The risk factors for sarcoma also include virus infection. Kaposi’s sarcoma, which affects the skin, mouth, and other tissues, consists of spindle-shaped cells and appears dark purplish due to the leakage of red blood cells from the heavily vascularized (enriched with blood vessels) tumors. A commonly associated event is the infection by HHV-8 (human gammaherpesvirus 8), a DNA virus with a large genome (~145 kilobases), which encodes the genes involved in cell cycle regulation (ex. cyclin D that complexes with cyclin-dependent kinase to phosphorylate the Rb protein), nucleotide biosynthesis (ex. thymidylate synthetase, dihydrofolate reductase), immune regulation, etc. (Moore et al., 2001; Gáspár et al., 2002). It may occur with weakened immunity, ex. transplantation patients taking immunosuppressive drugs to avoid rejection of transplanted organs, and cancer patients undergoing chemotherapy. Kaposi’s sarcoma has received wide publicity due to its frequent occurrence in HIV-1 (human immunodeficiency virus) infected patients due to the depletion of T helper cells (CD4-positive) and other types of immune cells.
In the adaptive (as opposed to innate) immune system, T helper cells play a significant role. Following the encounter with a pathogen (ex. virus, bacteria, fungus), the internalized pathogen is proteolytically processed and selected resultant peptides are presented by class II major histocompatibility complex in antigen processing cells (ex. dendritic cells, macrophages, B lymphocytes). The presented peptide may then get recognized by naïve ‘T helper cells’ expressing a cognate T cell receptor (specific for the presented peptide) plus the co-receptor CD4 (binds to a distinct site on class II MHC molecule). [Note: in the case of ‘cytotoxic T cells’, the co-receptor CD8 recognizes class I MHC molecule). The activated T helper cells contribute to immune function via the secretion of specific cytokines and are involved in the activation of macrophages, B lymphocytes, cytotoxic T cells, etc.
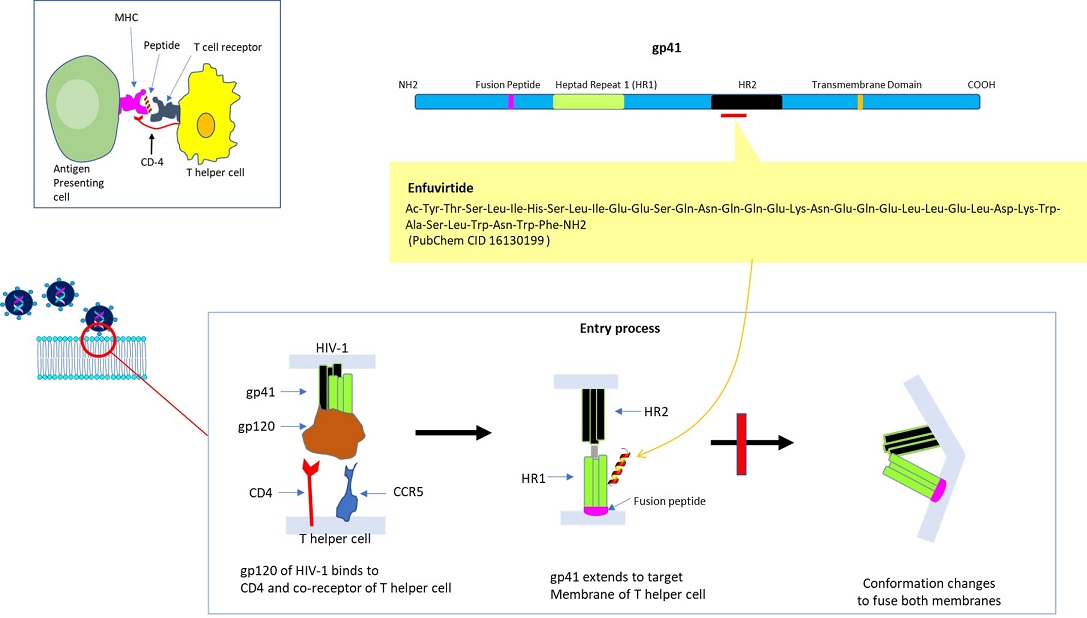
CD4 molecule present on the surface of T helper cells also serves as the receptor for HIV-1. HIV-1 is a negative-stranded RNA virus that relies on the virally encoded reverse transcriptase to convert its genomic RNA into cDNA, which is converted to double-stranded DNA by using ‘DNA-dependent DNA polymerase’ to synthesize its complementary strand. During the latent stage, double-stranded HIV-1 genomic DNA is integrated (provirus) into host cells’ chromosomal DNA (via integrase) after entering the nucleus. The translation of mRNAs transcribed from the provirus produces polypeptides necessary for assembling the HIV-1 virus.
A high level of errors introduced during the cDNA synthesis by reverse transcriptase, resulting in viruses with highly variant epitopes, has been the key mechanism through which HIV-1 has defied the attempt to suppress the pandemic (caused ~40 million deaths globally) via vaccination. Its high rate of evolution (ca. million times fast than the human genome) has also contributed to the emergence of resistant strains to antiretroviral drugs (ex. Inhibitors of reverse transcriptase).
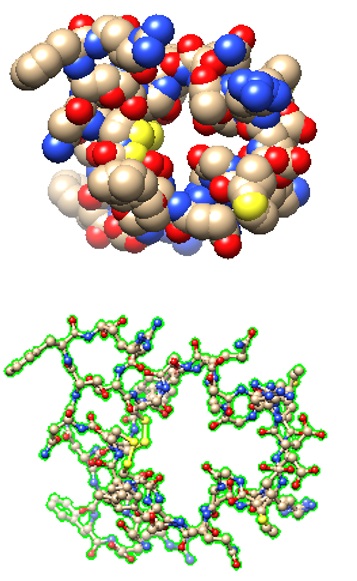
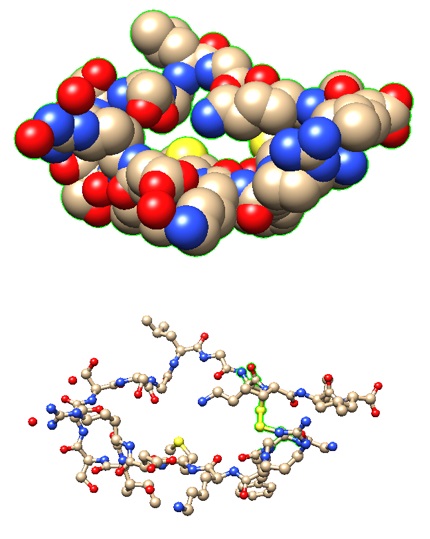
This has led to the devising of alternate peptide-based therapeutics to interfere with HIV-1 infection. Briefly, the entry of HIV-1 is a multi-step process, in which the initial stage (albeit not essential) may consist of the interaction of its Envelope protein (a heterodimer comprised of gp120 and gp41 proteins) with cell surface molecules, i.e. heparan sulfate, alpha4beta7 integrin, or pattern recognition receptor DC-SIGN (dendritic cell–specific intercellular adhesion molecular 3-grabbing non-integrin). The engagement of CD4 (of T helper cell) by gp120 (of HIV-1) induces a conformational change to expose the domain that interacts with the co-receptor, i.e. chemokine receptor CCR5 or CXCR4 (of T helper cell). Subsequently, the hydrophobic fusion peptide gp41 inserts into the cell membrane, followed by the folding of hinge regions (N-terminal helix ‘HR-N’ and C-terminal helix ‘HR-C’) to form a 6-helix bundle, fusing viral and cellular membranes to allow the entry of viral capsid (Wilen et al., 2012). As such, multiple steps could be targeted to disrupt HIV-1 internalization. The viral entry inhibitor Enfuvirtide (also called Fuzeon; F. Hoffmann-La Roche AG Pharmaceuticals) is a peptide consisting of 36 residues, which was approved by U. S. FDA (Food & Drug Administration) in 2003. The biomimetic peptide (derived from amino acids 643 -678 of gp41) binds to the prehairpin structure of HR-N to block the 6-helix bundle formation to suppress membrane fusion for HIV-1 entry (Greenberg et al., 2004). Its limitations include adverse side effects, inability to administer orally, and inflammatory response.
In a similar vein, various peptide drugs have been developed to block the infectivity of SARS-CoV-1, which caused the 2002-2004 SARS outbreak (Madhavan et al., 2021). A high degree of conservation between SARS-CoV-1 (caused SARS) and SARS-CoV-2 (caused the COVID pandemic in 2019) coronaviruses has inspired the development/testing of similarly designed peptides to suppress the infectivity of the latter albeit mostly in a preclinical setting (Shah et al., 2022).
The key to preventing an epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogs (over ~200) including bridged nucleic acid (BNA) in addition to mRNA synthesis. A number of options are available to label oligonucleotides (DNA or RNA) with fluorophoreseither terminally or internally as well as to conjugate to peptidesor antibodies. It provides custom conjugation of small molecules such as chemical drugs, metabolites and labeled compounds with synthetic or natural polymers (enzymes, peptide, protein, oligonucleotide, antibody, dendrimer, nanoparticle, etc). It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, the third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. It has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple-negative breast cancer. The BNA technology provides superior, unequaled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides. For therapeutic consideration, peptide synthesis or modifications may include labeling, conjugation, cyclization, incorporation of unusual amino acids, and modification of side chain and backbone.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/mrna.aspx
https://www.biosyn.com/bioconjugation.aspx
https://www.biosyn.com/tew/Design-Guidelines-for-BNA-based-Oligonucleotide-Probes.aspx#!
Peptide Modifications, Modified Peptide Synthesis - Bio-Synthesis (biosyn.com)
References
Gáspár G, Neyts J, et al. Human herpesvirus 8 gene encodes a functional thymidylate synthase. J Virol. 76:10530-2 (2002). PMID: 12239332
Greenberg ML, Cammack N. Resistance to enfuvirtide, the first HIV fusion inhibitor. J Antimicrob Chemother. 54:333-40 (2004). PMID: 15231762
Madhavan M, Mustafa S, et al. Exploring peptide studies related to SARS-CoV to accelerate the development of novel therapeutic and prophylactic solutions against COVID-19. J Infect Public Health. 14:1106-1119 (2021). PMID: 34280732
Moore PS, Chang Y. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 356:499-516 (2001). PMID: 11313008
Pondrom M, Brugières L, et al. Rhabdomyosarcoma associated with germline TP53 alteration in children and adolescents: The French experience. Pediatr Blood Cancer. 67(9):e28486 (2020). PMID: 32658383
Shah JN, Dua K, et al. Peptides-based therapeutics: Emerging potential therapeutic agents for COVID-19. Therapie. 77:319-328 (2022). PMID: 34689960
Shew JY, Lee WH, et al. Antibodies detecting abnormalities of the retinoblastoma susceptibility gene product (pp110RB) in osteosarcomas and synovial sarcomas. Oncogene Res. 205-214 (1989). PMID: 2740144
Wilen CB, Doms RW, et al. HIV: cell binding and entry. Cold Spring Harb Perspect Med. 2:a006866 (2012). PMID: 22908191


.jpg)
.jpg)
.jpg)





.jpg)


 ,
,



.jpg)