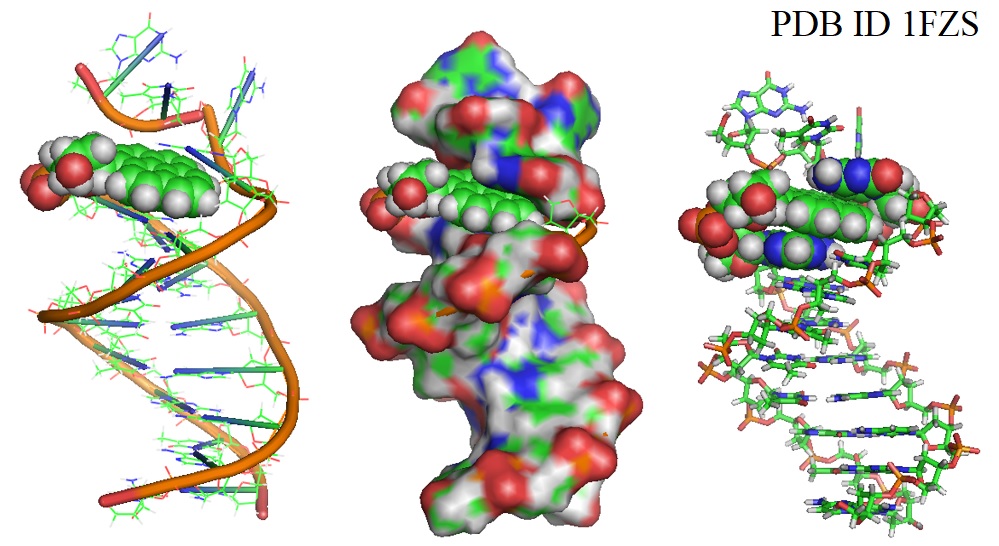
Of interest in this regard is herbal medicine, which contains additional ingredients besides the active component, whose impact on the therapeutic efficacy of the latter remains largely unexplored. The therapeutic potential of various plants and other biological resources has been recognized for thousands of years globally. This has led to the curating of therapeutic regimens dubbed 'ancient medicine' for various illnesses in multiple continents including Asia, Africa, the Americas, and Europe (Wargovich et al., 2001).
Numerous herbal products have been examined for potential anticancer effects through preclinical or clinical studies. These include mistletoe, green tea extract, phytoestrogens, and others. (Olaku et al., 2011). Sage (Salvia officinalis) is an evergreen shrub found in the Mediterranean region in Europe and elsewhere. Since ancient times, it has been used in religious rituals, cuisine, or herbal medicine (ex. fever). A preclinical study showed that essential oil distilled from sage leaves (containing multiple components including flavonoid glycosides, estrogenic substance, fumaric acid) suppresses the growth of colon cancer cells (Luca et al., 2020).
Traditional Chinese medicine (TCM) encompasses an extensive array of plants with potential therapeutic values for various illnesses including cardiovascular disease, inflammation, diabetes, and cancer (Luo et al., 2019). Malaria is a life-threatening disease caused by a single celled organism (protozoa) that infects and develops within red blood cells. Despite the centuries-old practice of treating malaria with chloroquine, the occurrence of resistant strains presented a significant clinical challenge in the 1960s. The discovery of artemisinin from the medicinal herb Artemisia annua by Y. Tu and colleagues (China Academy of Traditional Chinese Medicine; Nobel prize 2015) as a novel anti-malarial agent provided a necessary therapeutic relief to millions of afflicted patients globally (Miotto et al., 2015).
.jpg)
Among the best characterized traditional Chinese medicines is green tea extract (Wagner et al., 2011) EGCG (epigallocatechin-3-gallate) is the main polyphenol compound found in green tea extract, which has been extensively studied for its pharmacological properties (ex. anti-obesity, anti-inflammatory, anti-diabetic, anti-viral) though its excessive intake may lead to liver toxicity. The anti-oxidative property of EGCG results from the ability of the dihydroxy and trihydroxy groups present in its ring structure to scavenge radicals or chelate ions (Ouyang et al., 2020). Multiple reports have examined its growth inhibitory effect on breast cancer, lymphoma, gastric cancer, bladder cancer, etc. (Luo et al., 2019). More recently, its suppressive effect of various pathogenic viruses including COVID-19 coronavirus has been studied (Mhatre et al, 2020).
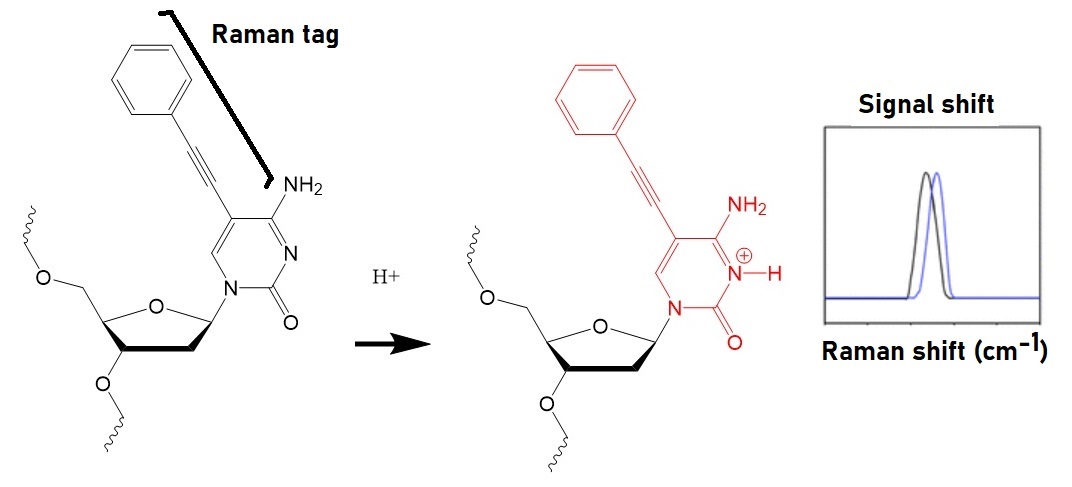
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA) in addition to mRNA synthesis. A number of options are available to label oligonucleotides (DNA or RNA) with fluorophoreseither terminally or internally as well as to conjugate to peptidesor antibodies. It provides custom conjugation of small molecules such as chemical drugs, metabolites and labeled compounds with synthetic or natural polymers (enzymes, peptide, protein, oligonucleotide, antibody, dendrimer, nanoparticle, etc). It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. It has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/mrna.aspx
https://www.biosyn.com/tew/peptide-mimetics.aspx
https://www.biosyn.com/bioconjugation.aspx
https://www.biosyn.com/tew/Dolastatin-Peptides.aspx#!
References
Calabria LM, Mabry TJ, et al. Triterpene saponins from Silphium radula. Phytochemistry. 69: 961-72 (2008). PMID: 18039545
Luo H, Wang Y, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med. 2019 Nov 6;14:48. PMID: 31719837
Luca T, Castorina S et al. Antiproliferative Effect and Cell Cycle Alterations Induced by Salvia officinalis Essential Oil and Its Three Main Components in Human Colon Cancer Cell Lines. Chem Biodivers. 17:e2000309 (2020). PMID: 32531144
Mhatre S, et al. Antiviral activity of green tea and black tea polyphenols in prophylaxis and treatment of COVID-19: A review. Phytomedicine. 2021. PMID: 32741697
Miotto O, Kwiatkowski DP, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. 47:226-34 (2015). PMID: 25599401
Molassiotis A, Kearney N., et al. Complementary and alternative medicine use in colorectal cancer patients in seven European countries. Complement Ther Med. 13:251-7 (2005). PMID: 16338195
Molassiotis A, Margulies A., et al. Complementary and alternative medicine use in lung cancer patients in eight European countries Complement Ther Clin Pract. 12:34-9 (2006). PMID: 16401528
Noll DM, Miller PS, et al. Preparation of interstrand cross-linked DNA oligonucleotide duplexes. Front Biosci. 9:421-37 (2004). PMID: 14766379
Oba K, Sakamoto J, et al. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 29:2739-45 (2009). PMID: 19596954
Olaku O, White JD. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer. 47:508-14 (2011). PMID: 21185719
Ouyang J, Huang J, et al. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid Med Cell Longev. 2020:9723686 (2020). PMID: 32850004
Siegel RL, Miller KD, et al. Cancer Statistics, 2021. CA Cancer J Clin. 71:7-33 (2021). PMID: 33433946
Wagner H., et al. Chromatographic Fingerprint Analysis of Herbal Medicines. In book: Chromatographic Fingerprint Analysis of Herbal Medicines (pp.203-209) January 2011. DOI:10.1007/978-3-7091-0763-8_18
Wargovich MJ, Woods C, et al. Herbals, cancer prevention and health. J Nutr. 131(11 Suppl):3034S-6S (2001). PMID: 11694643
Williams JD, Linse K, Mabry TJ, et al. The flavonoids and phenolic acids of the genus Silphium and their chemosystematic value. Nat Prod Commun. 4:435-46 (2009). PMID: 19413129


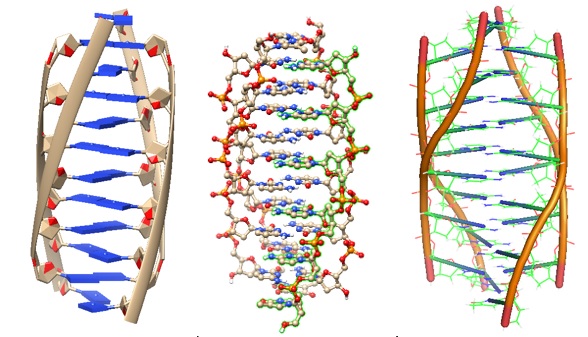
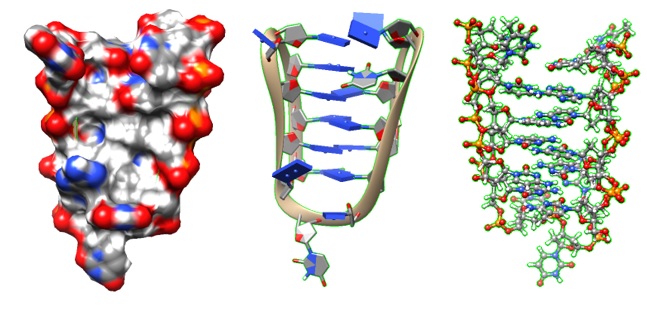
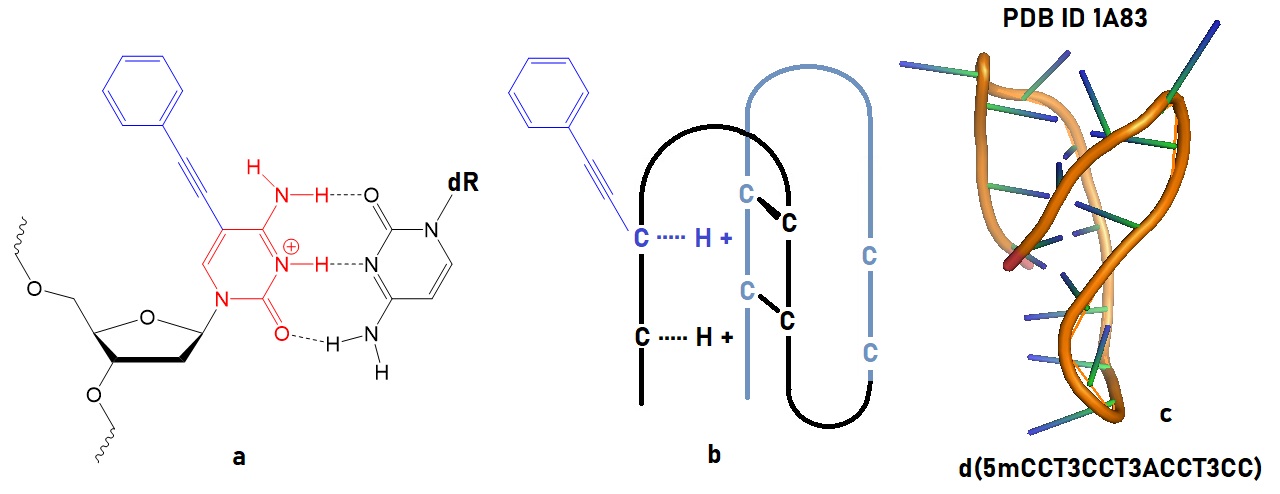


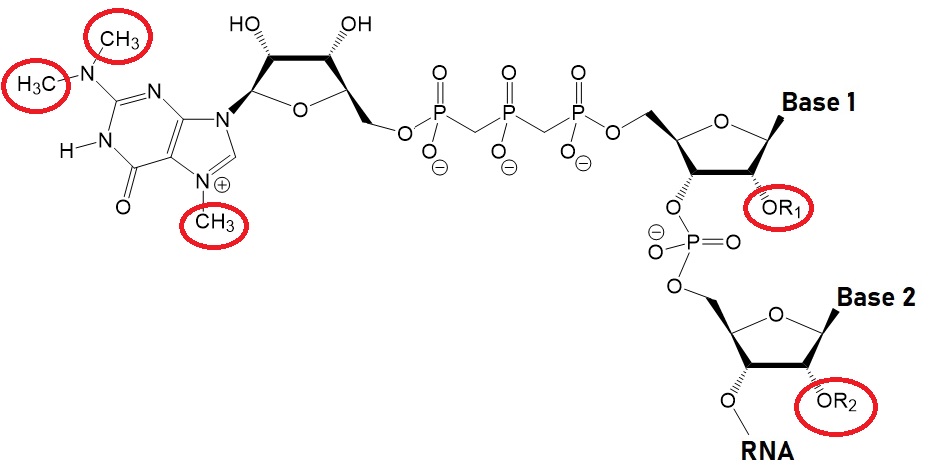




.jpg)

.jpg)
.jpg)





.jpg)
.jpg)
.jpg)