The protein musclin, known as osteocrin, regulates muscle metabolism and growth and is essential for exercise-induced cardiac protection. Inducing musclin signaling might serve as a novel therapeutic strategy for cardioprotection.
Exercise is the best way to promote physical and metabolic well-being. Exercise triggers cardiac conditioning, which is beneficial for healthy and diseased hearts. Muscles produce and secrete myokines. Myokines mediate local and systemic "crosstalk" during exercise to promote exercise tolerance and overall health, including cardiac conditioning. However, molecular mechanisms of exercise tolerance and its plasticity are only partially understood.
The myokine musclin acts as a ligand for natriuretic peptidereceptor NPR3/NPR-C and promotes bone growth and physical endurance in muscles.
In mammals, osteocrin regulates osteoblast differentiation and bone growth by binding to natriuretic peptide receptor NPR3/NPR-C, thereby preventing binding between NPR3/NPR-C and natriuretic peptides. In humans, osteocrin is a regulator of dendritic growth in the developing cerebral cortex in response to sensory inputs. Brain membrane depolarization induces the hormone. It inhibits dendritic branching in neurons of the developing cortex by binding to natriuretic peptide receptor NPR3/NPR-C, thereby preventing binding between NPR3/NPR-C and natriuretic peptides, leading to an increase in cyclic guanidine monophosphate (cGMP) production required to enhance physical endurance. Musclin (osteocrin) also may act as an autocrine and paracrine factor linked to glucose metabolism in skeletal muscle.
Using a mouse model, Harris et al., 2023, recently investigated the role and mechanisms by which the myokine musclin promotes exercise-induced cardiac conditioning. The infusion of the synthetic musclin peptide reproduced the cardioprotective benefits of exercise in sedentary wild-type (WT) and osteocrin knockout (Ostn-KO) mice.
The musclin peptide used for the study was the peptide with the sequence SFSGFGSPLDRLSAGSVEHRGKQRKAVDHSKKR.
Figure 1 shows the alignment of musclin protein sequences from rat, mouse, and human, with the sequence of the musclin peptide and sequences of several natriuretic peptides from atrial natriuretic peptide receptor-peptide complexes.
.jpg)
Figure 1: Alignment of sequences from rat, mouse, and human musclin with the sequences of natriuretic peptide A and B.
Relatively recently discovered, the musclin protein has been primarily studied in the context of skeletal muscle physiology. Musclin is a secreted protein predominantly expressed in skeletal muscle tissues. It belongs to the family of proteins known as "immunoglobulin-like domain-containing proteins." It is also called "IGFBP-rP10" (Insulin-like Growth Factor-Binding Protein-related Protein 10) due to its structural similarities to other proteins in the IGFBP family.
Thomas et al., 2003, utilized a viral-based signal-trap strategy to identify a novel gene the scientists called "osteocrin." A 1280-bp mRNA encoding osteocrin, producing a mature protein of 103 amino acids with a molecular mass of 11.4 kDa. The study identified two proteins in the medium of cells overexpressing osteocrin, a full-length 11.4 kDa protein and a processed approximately 5 kDa protein. Mutation of the 76KKKR79 dibasic cleavage site abolished the appearance of this smaller osteocrin fragment.
Nishizawa et al. 2004 identified musclin using a signal sequence trap of mouse skeletal muscle cDNAs. Musclin's copy or complementary DNA (cDNA) encoded 130 amino acids, including an NH2-terminal 30-amino acid signal sequence. The musclin protein contained a region homologous to the natriuretic peptide family and the amino acid sequence KKKR, a putative serine protease cleavage site similar to the natriuretic peptide family. Musclin cDNA-transfected mammalian cell cultures secreted the full-length musclin protein and a KKKR-dependent cleaved fragment. The musclin mRNA was expressed almost exclusively in the skeletal muscle of mice.
Recent research suggests that musclin has multiple functions in muscle metabolism. It enhances glucose uptake in muscle cells, promoting glucose utilization and improving insulin sensitivity. Musclin also appears to have an anti-inflammatory effect, as it can inhibit the production of specific pro-inflammatory cytokines. Additionally, studies have indicated that musclin may have anabolic effects on skeletal muscle. Musclin stimulates muscle protein synthesis and promotes muscle hypertrophy, potentially by activating signaling pathways involved in muscle growth.
The overexpression of musclin in muscle reduces cardiac dysfunction and myocardial fibrosis during pressure overload. Mechanistically, Musclin enhances the abundance of C-type natriuretic peptide (CNP), thereby promoting cardiomyocyte contractility through protein kinase A and inhibiting fibroblast activation through protein kinase G signaling.
However, more research is needed to fully understand its exact role in muscle physiology and its potential implications for various conditions such as metabolic disorders and muscle wasting diseases.
Atrial natriuretic peptide receptor/peptide complexes
Natriuretic peptides are a family of three structurally related hormones or paracrine factors. The cardiac atria, the two top chambers of the heart, and the ventricles, the two bottom chambers of the heart involved in pumping blood, secrete the atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). ANP signals in an endocrine and paracrine manner to decrease blood pressure and cardiac hypertrophy. BNP acts locally to reduce ventricular fibrosis. The C-type natriuretic peptide (CNP) primarily stimulates long bone growth but may also have other functions.
ANP and BNP activate the transmembrane guanylyl cyclase, natriuretic peptide receptor-A (NPR-A). CNP activates a related cyclase, natriuretic peptide receptor-B (NPR-B). Both receptors catalyze cGMP synthesis, which mediates the known effects of natriuretic peptides. A third natriuretic peptide receptor, natriuretic peptide receptor-C (NPR-C), clears natriuretic peptides from circulation through receptor-mediated internalization and degradation.
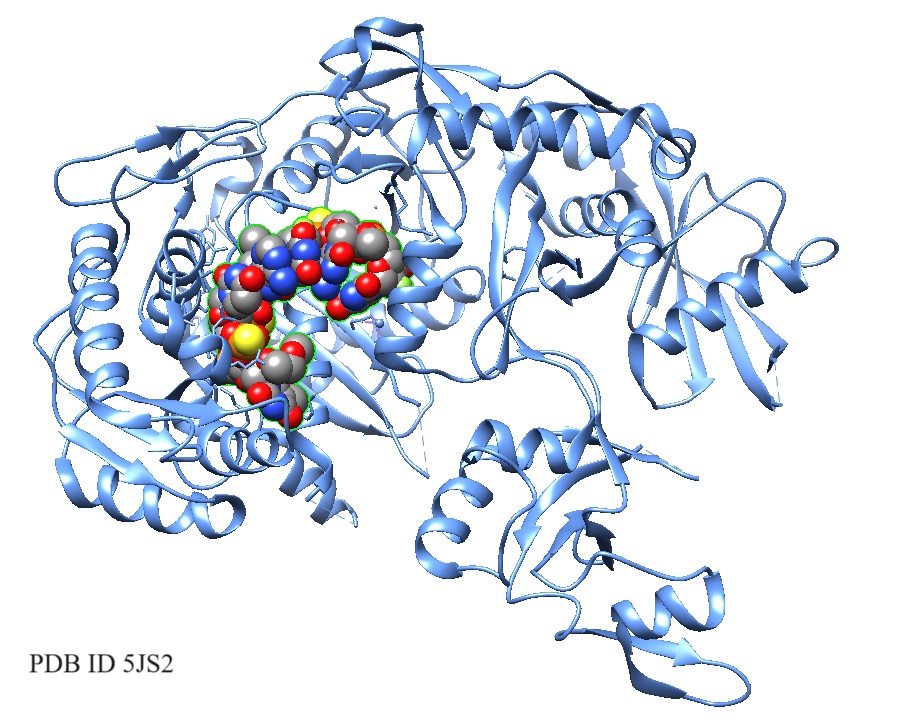
As of July 2023, several 3D structures of the atrial natriuretic peptide receptor are available in the PDB database, including 7BRK, 1YK1, 1YK0, 1JDP, and 1JDN.
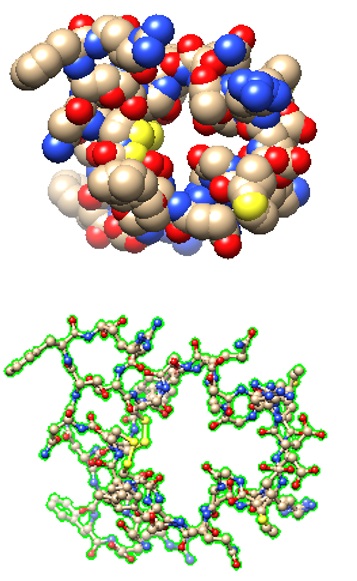
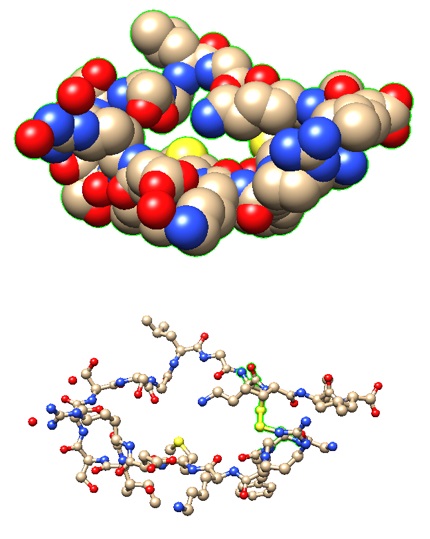
The structural models of two selected receptor-peptide complexes are shown below.
7BRK: Atrial Natriuretic Peptide Receptor complexed with deletion mutant of human Atrial Natriuretic Peptide [5-27] | Structural models of the human Atrial Natriuretic Peptide [5-27] as bound to the receptor. |
|
>pdb|7BRK|L Chain L, Natriuretic peptides A SSCFGGRMDRIGAQSGLGCNSFR |
1YK1: Structure of Natriuretic Peptide Receptor-C complexed with Brain Natriuretic Peptide | Brain natriuretic peptide |
|
>pdb|1YK1|E Chain E, Natriuretic peptides B GCFGRKMDRISSSSGLGCKVL |
Reference
Harris, Matthew P., Shemin Zeng, Zhiyong Zhu, Vitor A. Lira, Liping Yu, Denice M. Hodgson-Zingman, and Leonid V. Zingman.; 2023. "Myokine Musclin Is Critical for Exercise-Induced Cardiac Conditioning." International Journal of Molecular Sciences 24, no. 7: 6525. [IJMS]
Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, Kitamura T, Shimomura I.; Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004 May 7;279(19):19391-5. [PubMed]
Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM.; Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009;(191):341-66. [PMC]
Szaroszyk M, Kattih B, Martin-Garrido A, Trogisch FA, Dittrich GM, Grund A, Abouissa A, Derlin K, Meier M, Holler T, Korf-Klingebiel M, Völker K, Garfias Macedo T, Pablo Tortola C, Boschmann M, Huang N, Froese N, Zwadlo C, Malek Mohammadi M, Luo X, Wagner M, Cordero J, Geffers R, Batkai S, Thum T, Bork N, Nikolaev VO, Müller OJ, Katus HA, El-Armouche A, Kraft T, Springer J, Dobreva G, Wollert KC, Fielitz J, von Haehling S, Kuhn M, Bauersachs J, Heineke J.; Skeletal muscle derived Musclin protects the heart during pathological overload. Nat Commun. 2022 Jan 10;13(1):149. [PMC]
Thomas G, Moffatt P, Salois P, Gaumond MH, Gingras R, Godin E, Miao D, Goltzman D, Lanctôt C.; Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. J Biol Chem. 2003 Dec 12;278(50):50563-71. [PubMed]
Zunner BEM, Wachsmuth NB, Eckstein ML, Scherl L, Schierbauer JR, Haupt S, Stumpf C, Reusch L, Moser O.; Myokines and Resistance Training: A Narrative Review. Int J Mol Sci. 2022 Mar 23;23(7):3501. [PMC]
---...---












.jpg)