Oligonucleotide conjugation is predominantly carried out by use of a nucleophilic group on an oligonucleotide to react with an electrophilic group on a reporter molecule or a solid support. This is predominant approach because the common oligonucleotide deprotection is performed by base treatment e.g. ammonia, primary alkylamines or their combinations which are inherently nucleophilic. However, there are many situations when researchers need to introduce an electrophilic group into oligonucleotides and use it in the attachment method towards of a nucleophilic moiety.
In the case, when oligonucleotides have been used in that type of the bio-conjugation the activated carboxylate have been generated post-synthetically.2 Free carboxyl modified oligonucleotides can be activated by EDC in situ in the organic or aqueous conditions and subsequently conjugated to aminated counterpart (Fig. 1). However, in order to generate free carboxylate attached oligonucleotide using commercially available building blocks it requires cleavage of the ester bond with alkaline base4,5 before oligonucleotide base deprotection in concentrated ammonia, otherwise the ester will be converted into corresponding amide.
Figure 1.
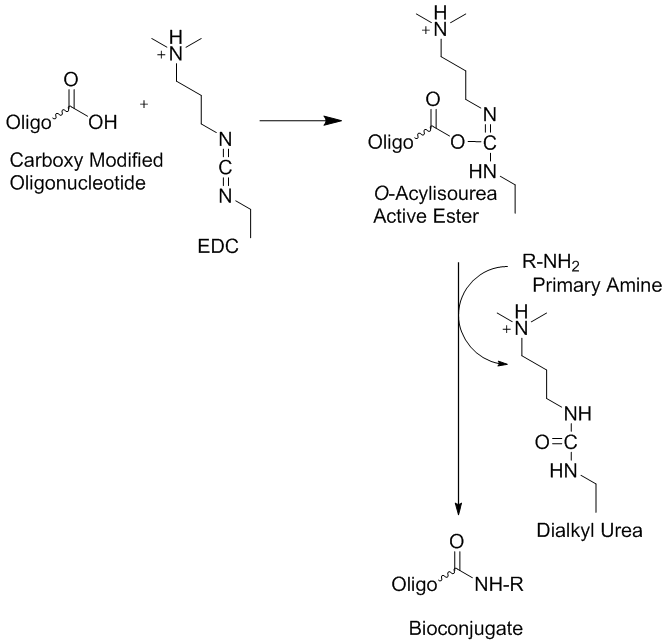

Using that strategy carboxyl modified oligonucleotides can be easily immobilized on solid support such as micro-array slides and various types of aminoalkylated beads.
Bio-synthesis offers not only wide varieties of 3’-, 5’- and internally carboxyl modified oligonucleotides and also their conjugates with peptides, proteins and antibodies.1
References:
1. http://www.biosyn.com/Bioconjugation.aspx
2. E. Jablonski, E. W.Moomaw, R. H.Tullis and J. L.Ruth Nucleic Acid Res., 1986, 14, 6115-6128.
3. J. D. Kahl and M. M. Greenberg J. Org. Chem., 1999, 64 (2), 507–510
4. A.V. Kachalova, T. S. Zatsepin, E. A. Romanova, D. A. Strelenko, M. J. Gait, T. S. Oretskaya Nucleosides, Nucleotides Nucleic Acids. 2000, 19, 1693-1707
5. T. P. Prakash, A. M. Kawasaki, E. A. Lesnik, S. R. Owens, M. Manoharan Org. Lett. 2003, 5, 403-406.