Shivarov et al. designed and validated a novel bead-based suspension assay using BNA[NC] probes for the Luminex Lab Scan 200 flow platform to detect and quantify somatic mutations in Leukemia!
-.-
Shivarov et al. recently develop and validated a novel bead-based suspension assay that uses BNA [NC] probes and the Luminex (USA) LabScan200 flow platform to allow the detection and quantification of DNMT3A p.R882C/H/R/S mutations in the blood of leukemia patients. The comparison with LNA based probes revealed the superior hybridization characteristics of the BNA based probes. The researchers were able to demonstrate for the first time the use of BNA[NC] probes coupled to fluorescently labeled beads for quantitative detection of DNMT3A R882 mutations. This type of assay has the potential for other molecular diagnostic applications as well.
-.-
Molecular diagnostics, techniques and methods used to analyze biological markers in genomic and proteomic research to diagnose and monitor disease, detect risk, and decide which therapies will work best for individual patients, offers the promise to enable and optimize personalized medicine.
Image may be NSFW.
Clik here to view.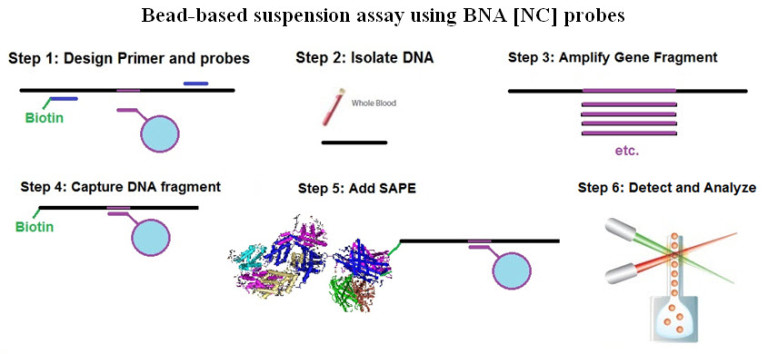
Bead-based suspension assay using BNA [NC] probes and the Luminex system to detect and quantify somatic mutations in leukemia. First, primers and probes are designed specific for the mutated sequence codons. Second, DNA is isolated from blood, and, third, amplified by PCR. Fourth, the biotinylated DNA fragments are captured with the capture probe connected to the beads, and, fifth, detected with a streptavidin-phycoerythrine (SAPE) complex. Sixth, the sample is analyzed using a Luminex instrument. The level of SAPE fluorescence is proportional to the amount of the captured DNA fragment.
-.-
Bead-based suspension assay using BNA [NC] probes and the Luminex system to detect and quantify somatic mutations in leukemia. First, primers and probes are designed specific for the mutated sequence codons. Second, DNA is isolated from blood, and, third, amplified by PCR. Fourth, the biotinylated DNA fragments are captured with the capture probe connected to the beads, and, fifth, detected with a streptavidin-phycoerythrine (SAPE) complex. Sixth, the sample is analyzed using a Luminex instrument. The level of SAPE fluorescence is proportional to the amount of the captured DNA fragment.
Leukemia is an often malignant disease of the blood that has the tendency to become progressively worse. This type of cancer of the blood or bone marrow is characterized by an abnormal increase of immature white blood cells. However, the term leukemia is also used to cover a whole spectrum of diseases affecting the blood, bone marrow, and lymphoid system, all known as hematological neoplasms. Leukemia is now considered to be a treatable disease. Several hematologic malignant tumors are characterized by genome instabilities. As identified by whole genome sequencing these cancer types frequently have between 10,000 and 100,000 mutations in their entire genomes and mutations in the human DNA methyl transferase 3A (DNMT3A) gene have now been identified in several blood diseases. The methyl-group transferring enzyme DNA methyltransferase 3A (DNMT3A) is one of two human de-novo DNA methyltransferases essential for the regulation of gene expression and mutations and deletions in this protein have been observed in acute myeloid leukemia (AML), Acute lymphoblastic leukemia (ALL), myelodysplastic sydromes and myeloproliferative neoplasms. Myeloid cells represent a prominent part of local inflammatory infiltrates in the central nervous system (CNS) and appear to strongly contribute to the local inflammatory milieu and the pathological outcome of diseases involving these cells.
In 2013 Kim et al. used PCR and direct sequencing to analyze mutations of DNMT3A amino acid residue R882 in 99 acute leukemia patients, including 57 AML patients, 41 ALL patients and a single biphenotypic acute leukemia (BAL) patient. The most common immunophenotype in BAL patients is defined by the coexpression of B-lymphoid and myeloid markers and less frequently, T-lymphoid and myeloid markers. BAL has a high incidence of clonal chromosomal abnormalities, the most common being the t(9;22) (q34;q11) (Ph chromosome) and structural abnormalities involving 11q23. Data are emerging that BAL has a negative prognosis in both children and adults and this may be related to the underlying chromosome abnormalities. The research group detected DNMT3A expression in mononuclear cells of the bone marrow in these patients and in normal individuals using real time quantitative polymerase chain reaction. Approximately 17.5% (10/57) of AML patients were found to exhibit DNMT3A mutations, and four missense mutations were observed in the DNMT3A mutated AML patients, including R882 mutations and a novel single nucleotide polymorphism resulting in a M880V amino acid substitution. It is now known that somatic heterozygous mutations of the DNA methyltransferase gene DNMT3A occur frequently in acute myeloid leukemia and other hematological malignancies. The majority (∼60%) of these affect a single amino acid, Arg882 (R882), located in the catalytic domain of the enzyme. In 2013, Kim et al. could show that exogenously expressed mouse Dnmt3a proteins that have the corresponding R878 mutations largely fail to mediate DNA methylation in murine embryonic stem (ES) cells but are capable of interacting with wild-type Dnmt3a and Dnmt3b. The coexpression of the Dnmt3a R878H (histidine) mutant protein resulted in inhibition of the wild-type Dnmt3a and Dnmt3b to methylate DNA in murine ES cells. In addition the expression of Dnmt3a R878H in ES cells containing endogenous Dnmt3a or Dnmt3b induced hypomethylation which suggests that the DNMT3A R882 mutations, in addition to being hypomorphic, have dominant-negative effects. The current literature suggests that that the presence of DNMT3A mutations is an adverse prognosis biomarker in adult acute myeloid leukemia and that the rapid detection of DNMT3A R882 codon mutations allows for the early identification of poor risk patients with acute myeloid leukemia.
Shivarov et al. designed and validated a novel bead-based suspension assay using BNA[NC] probes for the Luminex Lab Scan 200 flow platform to detect and quantify somatic mutations in Leukemia!
Reference
Soo Jin Kim, Hongbo Zhao, Swanand Hardikar, Anup Kumar Singh, Margaret A. Goodell, and Taiping Chen; A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. December 12, 2013; Blood: 122 (25).
Shivarov V, Ivanova M, Naumova E; Rapid Detection of DNMT3A R882 Mutations in Hematologic Malignancies Using a Novel Bead-Based Suspension Assay with BNA(NC) Probes. PLoS One. 2014 Jun 10;9(6):e99769. doi: 10.1371/journal.pone.0099769. eCollection 2014.
Image may be NSFW.
Clik here to view.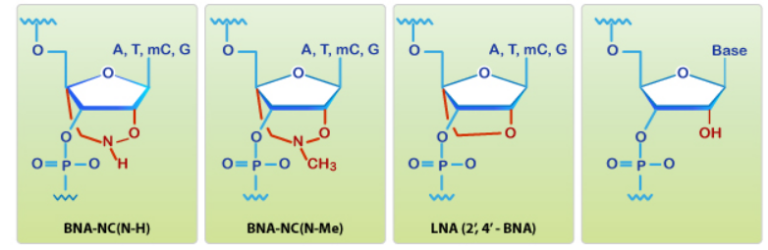
.
has 30 years experience in the analysis and synthesis of synthetic peptides, proteins, DNA and RNA oligonucleotides, bioconjugates and other biomolecules.
Call us at 1-800-227-0627
or visit our website at