Given the expanding scope of infection, the origin of recently emerged COVID-19 coronavirus remains a topic of interest to both scientists and the public at large. Identifying the origin of COVID-19 could be important as it may provide an opportunity to intervene. By targeting the source of the zoonotic transfer, transmission from the animal host to humans may be abrogated. In the case of SARS coronavirus, civet cat was identified as a potential intermediate host between bats and humans. Subsequently, their elimination was ordered in hopes of removing the reservoir host (Parry, 2004).From a pharmaceutical perspective, spike protein (S) has been the focus of research for both vaccine-based approaches as well as molecularly targeted approaches. For COVID-19 coronavirus, the gene encoding S protein is under intense evolutionary pressure. To adapt to a new host, spike protein must evolve to optimize its binding efficacy to human receptor(s). Being exposed at the virus exterior, spike protein is often the target of humoral response involving antibodies. To avoid viral elimination, the S gene must evolve further though mutation to escape recognition by the host immune system. Ultimately, these changes may have contributed to the emergence of a novel strain capable of the zoonotic transfer.
Previous works have identified 5 key residues in S protein of coronaviruses that are critical for binding to receptor (Wan et al., 2020). Subsequently, when the amino acids at the corresponding locations (plus one other residue) of COVID-19 (L455, F486, Q493, S494, N501 and Y505) and SARS coronavirus (Y442, L472, N479, D480, T487 and Y491) were compared, researchers at the Scripps Research Institute (United States) found only one of the six residues identical (Andersen et al., 2020). With Bat–RaTG13 coronavirus, which represents the closest relative (96% homology based on genomic sequence) (Zhou et al., 2020), COVID-19 also shared one identical amino acid (distinct from the above residue). Intriguingly, all six residues of S protein were found to be identical between COVID-19 and a coronavirus infecting Malayan pangolins (Manis javanica, a mammal with protective keratin scale) that may have been imported into Guangdong province, China. Hence, the possibility of genetic recombination occurring between Bat–RaTG13 coronavirus and the pangolin infecting strain was suggested.
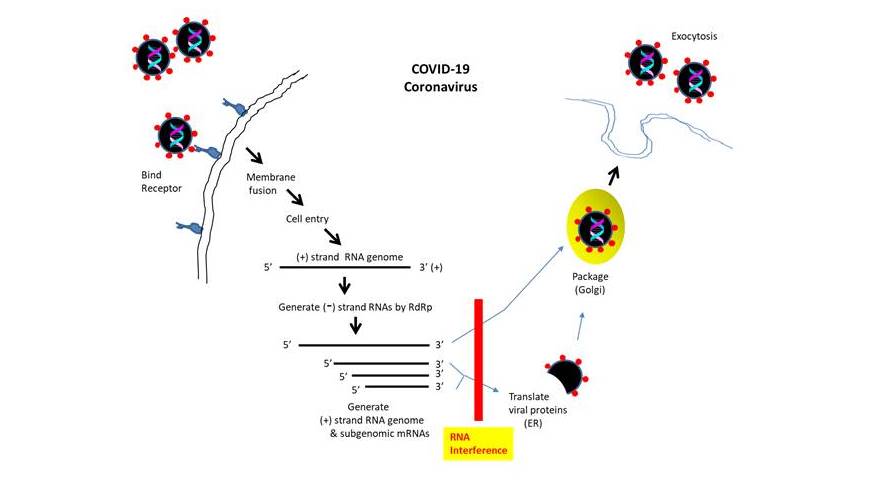
However, the above explanation alone may not suffice. Spike protein is comprised of S1 subunit (containing the receptor binding domain) and S2 subunit that contains heptad repeats HR1 and HR2 (which mediate the fusion of viral membrane with cell membrane for the entry of virus capsid containing its RNA genome). At the junction of S1 and S2 subunits of COVID-19, 4 novel residues (PRRA, proline-arginine-arginine-alanine) were inserted, which were missing in all other strains examined (SARS, Bat–RaTG13, pangolin-infecting coronaviruses, 2 other Bat-SARS related coronaviruses). With the presence of R (arginine) residue located adjacent to the inserted residues, it generated RRAR (argine-arginine-alanine-arginine), a polybasic cleavage site of furin. Furin is a serine endoprotease that cleaves precursor polypeptides (ex. pro-parathyroid hormone, pro-albumin, TGF beta-1 precursor) at the paired basic residues. To explain, the researchers posited that the insertion may have occurred during the human-to-human transfer.
![]()
Evident from the above analysis is the need to discriminate between closely related strains of coronaviruses with a high degree of accuracy. This is especially the case for determining COVID-19 infection status in individuals for epidemiological anlaysis. Different techniques can be deployed to diagnose COVID-19 at the RNA or protein level. For specificity, RT-PCR remains the ‘gold standard’ as it interrogates the genomic sequence albeit after reverse transcription. A commonly prescribed RT-PCR assay developed by CDC (Center for Disease Control) employs oligonucleotide primers and a TaqMan probe (dual-labeled with FAM and BHQ-1 fluorescent labels).
Of interest is a modification that provides even greater sensitivity to the above protocol. DNA binding proteins can be classified into ‘major groove binders’ versus ‘minor groove binders’. In general, proteins that interact with specific DNA sequences (ex. transcription factor) bind at the major groove to access the nucleotide bases. Minor groove binders (MGB) include Hoechst dye or DAPI (commonly used for DNA labeling) and the antibiotics (netropsin, distamycin). Duocarmycin is another minor groove binder that alkylates DNA (adenine) resulting in cell death, whose cytotoxicity has been exploited for cancer therapy (Tietze et al., 2009).
For RT-PCR, the minor groove binder, dihydrocyclopyrroloindole tripeptide (DPI3), has been used in TaqMan-MGB probe as its incorporation elevates Tm value, allowing for shorter probe (Kutyavin et al., 2000). Upon hybridization, the crescent shaped MGB (DPI3) folds back into the minor groove of the duplex formed by the terminal 5-6 bp of the oligonucleotide probe and the target DNA, which is primarily stabilized by van der Waal’s force (Kumar et al, 1998). Using TaqMan-MGB real-time RT-PCR assay, nearly 100-fold increase in sensitivity was achieved for diagnosing Muscovy duck reovirus (Zheng et al., 2020). TaqMan-MGB probe has been used for virus detection (ex. equine herpes virus 5, avian paramyxovirus type 1, infectious bursal disease virus, avian influenza virus) as well as SNP (single nucleotide polymorphism) genotyping (Zhang et al., 2017; Fratnik et al, 2010; Akkutay et al., 2014).
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. Antibody purification, characterization/quantification, modification and labeling are also offered. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophoreseither terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/tew/Minor-Groove-Binders-or-MGBs.aspx#!
https://www.biosyn.com/tew/Minor-Groove-Binder-Phosphoramidites.aspx#!
References
Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF.Andersen KG, et al. The proximal origin of SARS-CoV-2. Nat Med. 26:450-452 (2020). PMID: 32284615 doi: 10.1038/s41591-020-0820-9
Kumar S, Reed MW, Gamper HB Jr, Gorn VV, Lukhtanov EA, Foti M, et al. Solution structure of a highly stable DNA duplex conjugated to a minor groove binder. Nucleic Acids Res. 26:831-8 (1998). PMID: 9443977 doi: 10.1093/nar/26.3.831.
Kutyavin I, Afonina IA, Mills A, Hedgpeth J. 3’-minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Research. 2000; 28:655–61. PMID: 10606668 DOI: 10.1093/nar/28.2.655
Parry J. WHO queries culling of civet cats. BMJ 328(7432): 128 (2004). PMCID: PMC1150312 PMID: 14726333 doi: 10.1136/bmj.328.7432.128-b
Tietze LF, Krewer B.Tietze LF, et al. Novel analogues of CC-1065 and the duocarmycins for the use in targeted tumour therapies. Anticancer Agents Med Chem. 9:304-25 (2009). PMID: 19275523 doi: 10.2174/1871520610909030304
Wan Y, Shang J, Graham R, Baric RS, Li F.Wan Y, et al. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol 94:e00127-20 (2020). PMID: 31996437 doi: 10.1128/JVI.00127-20.
Zhang Z, Liu D, Sun W, Liu J, He L, Hu J, et al. Multiplex one-step Real-time PCR by Taqman-MGB method for rapid detection of pan and H5 subtype avian influenza viruses. PLoS One 12:e0178634. PMID: 28575115 doi: 10.1371/journal.pone.0178634. eCollection 2017.
Zheng M, Chen X, Wang S, Wang J, Huang M, et al. A TaqMan-MGB Real-Time RT-PCR Assay With an Internal Amplification Control for Rapid Detection of Muscovy Duck Reovirus. Mol Cell Probes (2020) Apr 17;101575. doi: 10.1016/j.mcp.2020.101575. (Online ahead of print)
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270-273 (2020). PMID: 32015507 doi: 10.1038/s41586-020-2012-7.
.jpg)





.jpg)






.png)

.jpg)


.jpg)


.png)
