Amino Acid Analysis to elucidate Tryptophan Pathways
By Klaus D. Linse
The Serotonin Pathway
![Tryptophan-Pathway]() Figure 1: The serotonin pathway: L-tryptophan is converted to serotonin (5-hydroxytryptamine or 5-HT) via 5-hydroxytryptophan which can be further metabolized to 5-hydroxyindole acetic acid.
Figure 1: The serotonin pathway: L-tryptophan is converted to serotonin (5-hydroxytryptamine or 5-HT) via 5-hydroxytryptophan which can be further metabolized to 5-hydroxyindole acetic acid.
![Tryptophan-Pathway]() Figure 2: The first steps of the kynurenine pathway are illustrated: L-tryptophan is metabolized into kynurenine via formylkynurenine.
Figure 2: The first steps of the kynurenine pathway are illustrated: L-tryptophan is metabolized into kynurenine via formylkynurenine.
![Tryptophan-Pathway]() Figure 3: The kynurenine pathway: Kynurenine, the central metabolite of the kynurenine pathway, resulting from the catabolism of L-tryptophan is further metabolized into several other metabolites. Many of these metabolites appear to have important functions in the central nervous system (CNS).
Figure 3: The kynurenine pathway: Kynurenine, the central metabolite of the kynurenine pathway, resulting from the catabolism of L-tryptophan is further metabolized into several other metabolites. Many of these metabolites appear to have important functions in the central nervous system (CNS).
Geier EG, Schlessinger A, Fan H, Gable JE, Irwin JJ, Sali A, Giacomini KM.; Structure-based ligand discovery for the Large-neutral Amino Acid Transporter 1, LAT-1. Proc Natl Acad Sci U S A.<http://www.ncbi.nlm.nih.gov/pubmed/23509259> 2013 Apr 2;110(14):5480-5. doi: 10.1073/pnas.1218165110. Epub 2013 Mar 18.
Hayashi K, Jutabha P, Endou H, Sagara H, Anzai N.; LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Immunol.<http://www.ncbi.nlm.nih.gov/pubmed/24038088> 2013 Oct 15;191(8):4080-5. doi: 10.4049/jimmunol.1300923. Epub 2013 Sep 13.
Rishabh Kapila, Kikeri Seetaramaih Nagesh, Asha Iyengar, Divyalakshmi Diabetes and Oral Changes: The Tryptophan Metabolism Link? Journal of Clinical and Diagnostic Research. 2012 May (Suppl-1), Vol-6(3):517-520.
Walter H. Kaye, Ursula F. Bailer, Guido K. Frank, Angela Wagner, Shannan E. Henry; Brain imaging of serotonin after recovery from anorexia and bulimia nervosa. Physiology & Behavior 86 (2005) 15 - 17.
ALFRED F. MICHAEL, KEITH N. DRUMMOND, DORIS DOEDEN, JOHN A.
ANDERSON, AND ROBERT A. GOOD; Tryptophan Metabolism in Man. Journal of Clinical Investigation Vol. 43, No. 9, 1964.
Web links
The "Tryptophan metabolism - Reference pathway" - http://www.genome.jp/kegg-bin/show_pathway?map00380+C00024
Tryptophan Metabolism - http://pathman.smpdb.ca/pathways/SMP00063/pathway
MetaCyc, a database of nonredundant, experimentally elucidated metabolic pathways. - http://metacyc.org/
By Klaus D. Linse
Modern automated amino acid analysis is considered to be the standard tool for quantitative analysis of metabolites of the tryptophan pathways. All metabolites containing primary and secondary amino groups are amenable to multiple amino acid analysis (AAA) methods. However, metabolites containing chromophores in their core structure can also be analyzed using high-performance liquid-chromatography (HPLC) and ultraviolet (UV) detection with fixed wavelength, variable wavelength or diode array detectors.
Tryptophan, in many organisms, is the precursor molecule for other biologically essential compounds such as niacin, present in most eukaryotes; indole acetic acid, found in most plants; and indole, present in many bacteria. Nutritional studies have established the role of tryptophan as a precursor in eukaryotic nicotinamide adenine dinucleotide (NAD+) biosynthesis. NAD+ is a coenzyme found in all living cells. For example, it was noticed that pellagra, a disease caused by deficiencies of nicotinamide or niacin can be avoided or cured by the addition of tryptophan or niacin to the patient's diet. Many other studies established tryptophan as a precursor of NAD in many animal and plants. Michael et al. in 1964 published a paper describing the "Tryptophan Metabolism in Man." The metabolic pathway was established by generating kinetic and quantitative data by the analysis of urinary excretions of 13 tryptophan metabolites from children and adults. The analytical biochemical method used was an early form of liquid chromatography called "elution column chromatographic method." A dowex 50 W-X12 column (200 to 400 mesh) was used for this purpose.
In humans, tryptophan is transported across the blood-brain barrier with the help of the large-neutral amino acid transporter. The large-neutral amino acid transporter 1 is a sodium-independent exchanger found in the brain, testis, and placenta. The transporter molecule mediates the transport of large-neutral amino acids, for example, tyrosine, and thyroid hormones, such as triiodothyronine, across the cell membrane. Recent research uncovered that the L-type amino acid transporter 1 (LAT1) is a major transporter for essential amino acids into activated human T cells, indicating that the activated immune system needs a sufficient supply of amino acids to function well. Tryptophan contains an indole core in its structure and is metabolized through several different biochemical pathways. In humans, tryptophan is an essential amino acid that is metabolized by several pathways. The major pathways are: the serotonin and kynerunine pathways. Tryptophan is the precursor molecule of serotonin, 5-hydroxyindole acetic acid and other indolic acids, such as 3-indole acetic acid. Degradation of tryptophan requires several enzymes called oxygenases. The enzyme tryptophan 2,3-dioxygenase cleaves the pyrrole ring, and kynurenine 3-monooxygenase hydroxylates the remaining benzene ring (adds an hydroxyl group to the benzene ring). Next, an alanine moiety is removed and the 3-hydroxyanthranilic acid is cleaved by another enzyme called a dioxygenase and subsequently processed to acetoacetyl Coenzyme A. Parts of the different metabolic pathways of tryptophan as described by Michael et al. in 1964 are outlined below. In addition, more details about metabolic pathways can be reviewed in standard biochemistry books such as the Lehniger and others as well as some web links listed under references.
The Serotonin Pathway
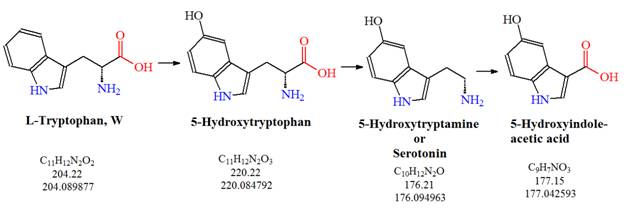
According to a paper published by Michael et al. in 1964, serotonin is synthesized from L-tryptophan via a short metabolic pathway in humans and animals. The two enzymes tryptophan hydroxylase (TPH) and amino acid decarboxylase (DDC) are needed for this pathway to function. L-tryptophan is converted to serotonin (5-hydroxytryptamine or 5-HT) via 5-hydroxytryptophan and can be further metabolized to 5-hydroxyindole acetic acid. It was found that the TPH-mediated reaction is the rate-limiting step in the pathway. The serotonin pathway helps to regulate mood control and dysregulation of the pathway can lead to obsessive-compulsive disorders such as anxiety disorders or depression.
The Kynurenine Pathway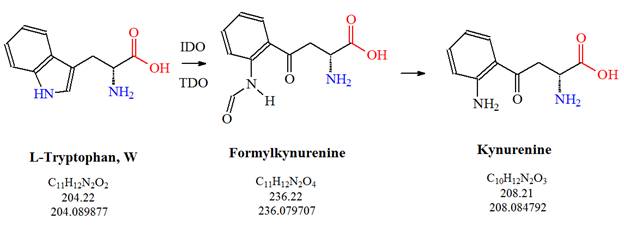
Approximately 95 to 99% of dietary tryptophan not used in protein synthesis is metabolized by the kynurenine pathway. L-tryptophan is enzymatically converted to kynurenine via formylkynurenine. The enzyme indoleamine 2,3-dioxygenase (IDO), a heme enzyme, catalyzes the first and rate-limiting step in tryptophan catabolism to N-formyl-kynurenine. The enzyme can act on multiple tryptophan substrates such as D-tryptophan, L-tryptophan, 5-hydroxy-tryptophan, tryptamine, and serotonin and is thought to play a role in a variety of pathophysiological processes such as antimicrobial and antitumor defense, neuropathology, immunoregulation, and antioxidant activity. IDO is expressed in dendritic cells, monocytes, astrocytes, microglia, microvascular endothelial cell, and macrophages and modulates T-cell behavior by its peri-cellular catabolization of the essential amino acid tryptophan. [Source: http://www.ncbi.nlm.nih.gov/gene/3620]. Tryptophan 2,3-dioxygenase (TDO2) in humans, another heme enzyme, also catalyzes the first and rate-limiting step of the kynurenine pathway. It is thought that increased activity of the protein followed by subsequent kynurenine production may also play a role in cancer through the suppression of antitumor immune responses, and single nucleotide polymorphisms in this gene may be associated with autism. [Source: RefSeq, Feb 2012].TDO is expressed in liver, kidney and brain cells. Both enzymes appear to be activated by stress hormones. Kynurenine inhibits tryptophan transport via the blood-brain barrier and stimulates IDO activity.
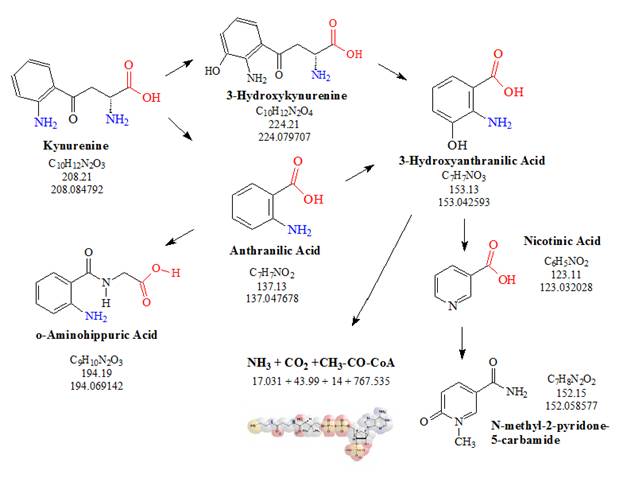
Nicotinamide (niacinamide or nicotinic acid amide, the amide of nicotinic acid), and niacin (vitamin B3 or nicotinic acid), are synthesized in the liver from tryptophan, and distributed to other tissues. The role of tryptophan as a precursor in eukaryotic nicotinamide adenine dinucleotide (NAD) biosynthesis was first suggested by nutritional studies in which humans stricken with pellagra, a nicotinamide (niacine) deficiency disease, recovered after the addition of tryptophan or niacin to their diets. Other studies established tryptophan as a precursor of NAD in many animal and plant systems. In eukaryotes, the de novo synthesis pathway for NAD starts with tryptophan. The pathway is closely related to the catabolic pathway of tryptophan, suggesting an evolutionary link between the two.
Roughly 60 mg of tryptophan are equivalent to 1 mg nicotinamide in humans, but the exact amounts may vary for individuals. Suboptimal levels of vitamin B-6, correlating with low plasma pyridoxal 5'-phosphate (PLP) concentrations, which is considered to be the active form of vitamin B-6, are associated with an increased risk of vascular disease, a pathological state of large and medium sized muscular arteries, triggered by endothelial cell dysfunction, and some cancers. PLP plays many roles in the human metabolism but also serves as a coenzyme in the catabolism of tryptophan. In addition it was also proposed that diabetes could also result, in part, in the activation of the tryptophan metabolism which reduces the plasma tryptophan levels and elevates the kynurenine metabolite levels. Several products of the kynurenine pathway are reported to have anti insulin action.
Tryptophan metabolites in the kynurenine pathway play important roles in several fundamental biological processes, including neuronal excitability, antioxidant status, ultraviolet protection, cell growth and cell division, and some kynurenine derivatives present in the saliva are also implicated in the onset and the development of periodontal disease in humans. In the hypothalamus, which secretes several releasing factors controlling the secretion of hormones from the anterior pituitary gland, serotonin is one of the many neurotransmitters that take part of the hypothalamic control of pituitary secretion, such as the regulation of adrenocorticotropin, prolactin and growth hormone secretion. However, the exact role serotonin plays in regulating stress-induced signals or the circadian periodicity of the hypothalamic-pituitary-adrenal axis is still unclear and may need to be elucidated further. Alternative tryptophan metabolizing pathways are the conversion of tryptophan to 5-hydroxytryptamine (5-HT) and then to melatonin, or to tryptamine and then to kynuramines (or kynurenamines).
Kaye et al. in 2005, showed that it is now possible to investigate the potential relationship of 5-HT functional activity and stereotypic core symptoms that influence and regulate eating behavior, temperament and personality, body image distortion, cognition, physical exercise, as well as age of onset and gender in humans and animals using modern brain imaging methods. The researchers suggest that positron emission tomography (PET) with radioligands offer opportunities to directly characterize brain 5-HT pathways and their relationship with behavior. They suggested that the disorders anorexia nervosa and bulimia nervosa can be studied this way.
Anorexia nervosa (AN) is an eating disorder characterized by immoderate food restriction and irrational fear of gaining weight, including a distorted body self-perception. The disorder typically results in excessive weight loss and usually occurs more in females than in males. Bulimia nervosa (BN) is an eating disorder characterized by binge eating and purging. In this disorder a large amount of food is consumed in a short amount of time followed by an attempt to get rid of the consumed food. This is typically done by vomiting, taking a laxative, diuretic, or stimulant, and/or excessive exercise. The cause is thought to be an extensive concern for body weight. Both disorders share common symptoms such as extremes of food consumption, body image distortion, anxiety and obsessions, including ego-syntonic neglect. Most patients that are diagnosed to have this disorder will respond negatively to suggestions by concerned well meaning friends or family members that they are ill and are deeply distraught by efforts to interrupt their ongoing quest toward further weight loss. It is possible that these symptoms reflect disturbances in brain functions linked to 5-HT that contribute to the pathophysiology of these illnesses.
All metabolites of these pathways containing primary and secondary amino groups can be analyzed using standard amino acid analysis (AAA) methods. Metabolites containing chromophores in their core structure can be analyzed using high-performance liquid-chromatography (HPLC) and ultraviolet (UV) detection with fixed wavelength, variable wavelength or diode array detectors. Other methods such as HPLC connected to refraction or amperometric detection have been used in the past as well. More recently new hyphenated techniques such as mass spectrometry based techniques have also been reported to be very useful for this type of analysis.
Please visit our website for more information on Amino Acid Analysis
ReferencesPlease visit our website for more information on Amino Acid Analysis
Vanessa R. da Silva, Luisa Rios-Avila, Yvonne Lamers, Maria A. Ralat, Øivind Midttun, Eoin P. Quinlivan, Timothy J. Garrett, Bonnie Coats, Meena N. Shankar, Susan S. Percival, Yueh-Yun Chi, Keith E. Muller, Per Magne Ueland, Peter W. Stacpoole, and Jesse F. Gregory III; Metabolite Profile Analysis Reveals Functional Effects of 28-Day Vitamin B-6 Restriction on One-Carbon Metabolism and Tryptophan Catabolic Pathways in Healthy Men and Women. J. Nutr. 2013 143: 1719-1727; first published online August 21, 2013. doi:10.3945/jn.113.180588.
Fukuwatari T, Shibata K.; Effect of nicotinamide administration on the tryptophan-nicotinamide pathway in humans. Int J Vitam Nutr Res.<http://www.ncbi.nlm.nih.gov/pubmed/18271280> 2007 Jul;77(4):255-62.
Geier EG, Schlessinger A, Fan H, Gable JE, Irwin JJ, Sali A, Giacomini KM.; Structure-based ligand discovery for the Large-neutral Amino Acid Transporter 1, LAT-1. Proc Natl Acad Sci U S A.<http://www.ncbi.nlm.nih.gov/pubmed/23509259> 2013 Apr 2;110(14):5480-5. doi: 10.1073/pnas.1218165110. Epub 2013 Mar 18.
Hayashi K, Jutabha P, Endou H, Sagara H, Anzai N.; LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Immunol.<http://www.ncbi.nlm.nih.gov/pubmed/24038088> 2013 Oct 15;191(8):4080-5. doi: 10.4049/jimmunol.1300923. Epub 2013 Sep 13.
Rishabh Kapila, Kikeri Seetaramaih Nagesh, Asha Iyengar, Divyalakshmi Diabetes and Oral Changes: The Tryptophan Metabolism Link? Journal of Clinical and Diagnostic Research. 2012 May (Suppl-1), Vol-6(3):517-520.
Walter H. Kaye, Ursula F. Bailer, Guido K. Frank, Angela Wagner, Shannan E. Henry; Brain imaging of serotonin after recovery from anorexia and bulimia nervosa. Physiology & Behavior 86 (2005) 15 - 17.
ALFRED F. MICHAEL, KEITH N. DRUMMOND, DORIS DOEDEN, JOHN A.
ANDERSON, AND ROBERT A. GOOD; Tryptophan Metabolism in Man. Journal of Clinical Investigation Vol. 43, No. 9, 1964.
Gregory F. Oxenkrug, MD, PhD; Tryptophan-Kynurenine Metabolism as a Common Mediator of Genetic and Environmental Impacts in Major Depressive Disorder: The Serotonin Hypothesis Revisited 40 Years Later. Isr J Psychiatry Relat Sci. 2010; 47(1): 56-63. <http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&retmode=ref&cmd=prlinks&id=20686200> PMCID: PMC3021918, NIHMSID: NIHMS261678.
Web links
The "Tryptophan metabolism - Reference pathway" - http://www.genome.jp/kegg-bin/show_pathway?map00380+C00024
Tryptophan Metabolism - http://pathman.smpdb.ca/pathways/SMP00063/pathway
MetaCyc, a database of nonredundant, experimentally elucidated metabolic pathways. - http://metacyc.org/