The potential of developing the RNA interference-based therapy for various infectious diseases or genetic disorders (ex. neurodegenerative disorders, cancer) is increasingly being explored. The siRNA technology involves introducing short dsRNA (~21 bp in length with 2 nucleotide 3’-overhang) into cells. Upon incorporation into the silencing complex RISC, the sense strand is removed typically. The remaining antisense strand guides RISC to target mRNA. Following the cleavage of the target mRNA at a discrete position, the resulting 5’ fragment and 3’ fragment are degraded by exosome and 5'-3' exoribonuclease 1 (XRN1), respectively. Its catalytic nature allows the degradation process to be repeated with additional mRNA targets. For pharmacological application, various issues such as stability, delivery, renal clearance and immune response are being addressed.
In 2018, Food and Drug Administration (FDA) approved the first RNA interference inducing drug Onpattro. Onpattro (also known as Patisiran) was developed by Alnylam Pharmaceuticals, Inc. to treat hereditary transthyretin-mediated amyloidosis (hATTR), a neurodegenerative disorder causing polyneuropathy (dysfunction of peripheral nerves). This rare but life-threatening disease is caused by the deposition of circulating transthyretin (TTR) amyloid in peripheral nerve, heart, kidney, gastrointestinal tract, etc., causing sensorimotor deterioration and various other symptoms including heart failure. Most hereditary cases are heterozygous for TTR mutation, and both mutant and normal TTR are found in the amyloid deposits (Coelho et al., 2013).
Onpattro (Patisiran) is a 21-mer double-stranded small interfering RNA (siRNA) oligonucleotide that targets 3’-UTR (untranslated region) shared by both normal and mutant TTR mRNAs. It incorporates 2’-O-methylcytidine and 2’-O-methyluridine at specific locations, with 2´-deoxythymidine dinucleotide overhangs at both 3´ ends. The RNA duplex is encapsulated in a cationic lipid nanoparticle, which is opsonized with ApoE (apolipoprotein E) to facilitate binding to ApoE receptor present on hepatic cell surface for uptake via endocytosis (Coelho et al., 2013). Phase 3 clinical trial demonstrated that Onpattro improves the symptoms of adult patients suffering from the hereditary transthyretin amyloidosis (Adams et al., 2018). One caveat is that the prescription cost for Onpattro could be quite high.
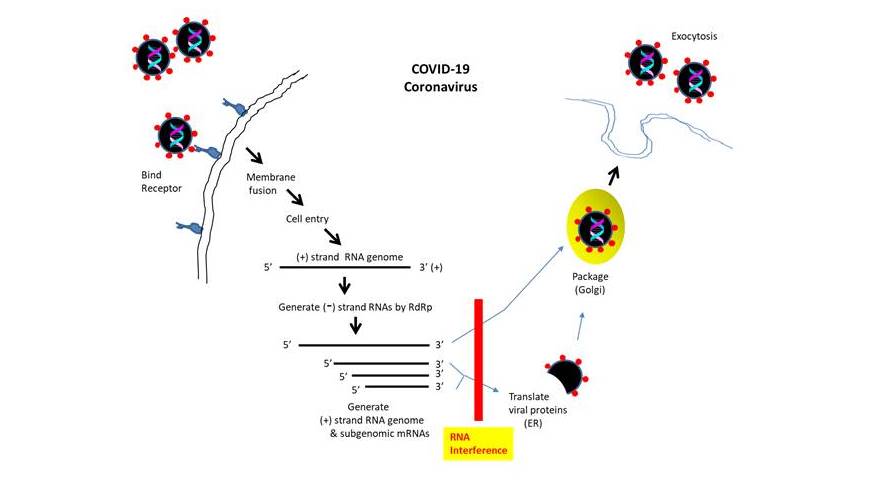
These advances have set the stage for the development of RNA interference therapy for other disorders such as cancer, diabetes, cardiac disease, etc. Additionally, the potential of utilizing siRNA oligonucleotides to treat infectious diseases (ex. hepatitis B virus) has been investigated. Previously, for SARS coronavirus, siRNAs targeting the regions in the genome encoding spike protein and ORF1b (NSP12) have been designed (Li B et al., 2005). Another report showed that siRNA targeting the ‘Leader’ sequence could suppress the replication of SARS coronavirus (Li T et al., 2005).
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/sirna-overview.aspx
References
Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 379:11-21 (2018). PMID: 29972753 doi: 10.1056/NEJMoa1716153.
Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev. 67:657-85 (2003). PMID:14665679 DOI: 10.1128/mmbr.67.4.657-685.2003
Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 369:819-29 (2013). PMID: 23984729 doi: 10.1056/NEJMoa1208760.
Cui L, Wang H, Ji Y, Yang J, Xu S, Huang X, Wang Z, et al. The Nucleocapsid Protein of Coronaviruses Acts as a Viral Suppressor of RNA Silencing in Mammalian Cells. J Virol 89:9029-43 (2015). PMID: 26085159 PMCID: PMC4524063 DOI: 10.1128/JVI.01331-15
Li BJ, Tang Q, Cheng D, Qin C, Xie FY, Wei Q, et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med 11:944-51 (2005). PMID: 16116432 PMCID: PMC7095788 DOI: 10.1038/nm1280
Li LC. "Small RNA-Mediated Gene Activation". RNA and the Regulation of Gene Expression: A Hidden Layer of Complexity. Caister Academic Press. ISBN 978-1-904455-25-7 (2008).
Li T, Zhang Y, Fu L, Yu C, Li X, Li Y, et al. siRNA targeting the leader sequence of SARS-CoV inhibits virus replication. Gene Ther. 12:751-61 (2005). PMID: 15772689 DOI: 10.1038/sj.gt.3302479
Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 42:217-39 (2013). PMID: 23654304 PMCID: PMC5895182 doi: 10.1146/annurev-biophys-083012-130404.