The transcription factor p53 regulates many cellular processes, including cell-cycle progression, apoptosis, senescence, DNA repair, and metabolism. Also, p53 is a potent tumor suppressor. Mice that do not have p53 develop normally but develop a variety of tumors. The gene encoding p53, TP53, is deleted or mutated in ~50% of human cancers. When p53 is mutated or erased, the protein no longer functions as a tumor suppressor. Since p53 is essential for the regulation of many cellular processes, p53 levels and activity need to be tightly controlled.
The murine double minute 2 (MDM2) oncogene is a primary regulator and inhibitor of p53. MDM2 inhibits the function of p53 via multiple mechanisms by direct protein-protein interaction through an autoregulatory feedback loop. MDM2 functions as an effective p53 antagonist or inhibitor in cells through direct interaction. Therefore, molecules that block the MDM2–p53 protein-protein interaction can lead to an increase of p53 protein and transcriptional activation of p53. Activating the tumor suppressor function of p53 is thought to have therapeutic potential for the treatment of human cancers retaining wild-type p53. Biochemical studies and the availability of a high-resolution cocrystal structure of MDM2 in complex with a p53 peptide enabled the design of molecular inhibitors blocking the MDM2-p53 interaction.
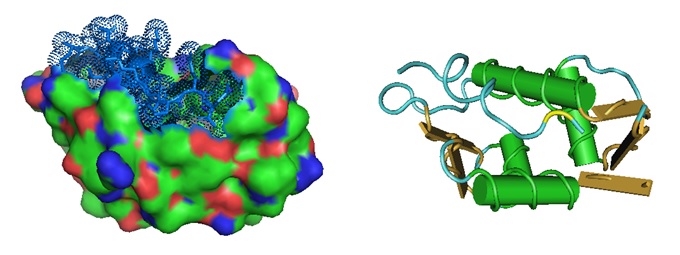
Figure 1: Structural models for the MDM2 oncoprotein in complex with a p53 tumor suppressor peptide. The image on the left shows the surface model, and the image on the right shows the ribbon model of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain, PDB 1YCR.
The crystal structure illustrates the oncogenic p53/MDM2 interaction, in which an N-terminal α-helix of the tumor suppressor p53 binds a hotspot on MDM2. MDM2 is a E3 ubiquitin ligase that downregulates p53 that is overexpressed in some cancers. Numerous peptide therapeutics have already been developed to target this interaction.
Miyachi et al. in 2009 suggested since p53 and MDM2 play important roles in tumor development and growth, inhibition of the MDM2-p53 pathway to consider as a promising therapeutic target for malignancies with wild-type p53. Inhibition of MDM2 expression using antisense oligonucleotides was shown to activate p53 and to suppress tumor growth in mouse xenograft models. However, it is still challenging to apply antisense therapy in clinical settings. Hence, the development of molecular inhibitors is also needed.
The small-molecule inhibitor nutlin-3 restored the p53 pathway in rhabdomyosarcoma (RMS) cell lines with wild-type p53. Miyachi, therefore, suggested that p53 restoration therapy is a potential therapeutic strategy for refractory RMS with wild-type p53.
An altogether different approach is the use of stabled peptides for the inhibition of protein-protein interactions. When cyclized peptides mimic native binding motifs, these peptides can inhibit protein-protein interactions and therefore the function of a protein. Macrocyclization, as used in stapled peptides, is a strategy for the synthesis of peptides with stable secondary structures. Cyclization can stabilize native bioactive conformations in peptides derived from proteins. Peptide mimicking native binding motifs can inhibit protein-protein interactions.
Recently, Lau et al. reported the design of stabled peptides acting as macrocyclic alpha-helical inhibitors of protein-protein interactions. The research group used a stabling technique based on a double strain-promoting azide-alkyne reaction. In their proof of concept experiment, MDM2-binding peptides were stapled in parallel, directly in the cell culture medium, and evaluated using a p53 reporter assay. For the double strain-promoting azide-alkyne reaction, Lau et al. prepared a strained diyne and tested the reaction using Fmoc-azido-homoalanine which resulted in the expected product. See figure 2.
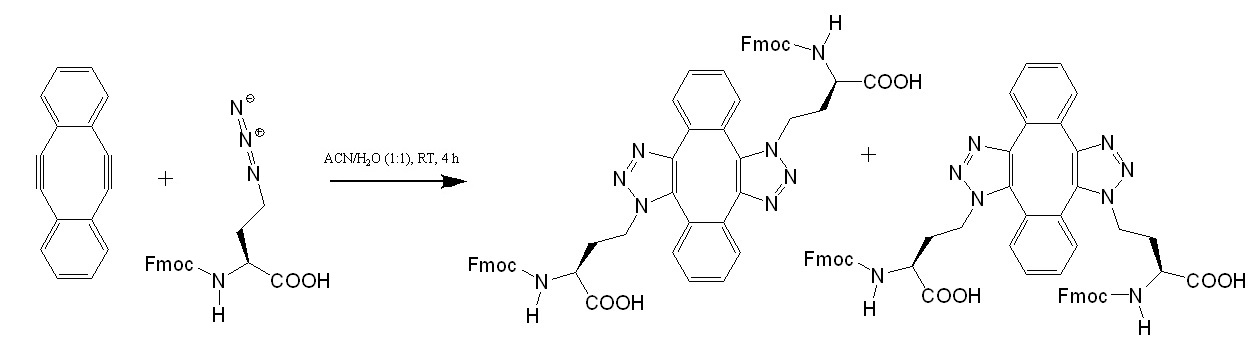
Figure 2: Strain-promoted azide-alkyne cycloaddtion (SPAAC).
Next, the stapling of a p53-derived diazidopeptide with the diyene linker in 1:1 H2O/tBuOH allowed the synthesis of the stapled peptide. However, minor byproducts of the same mass indicated that a modified peptide with a different conformation was also observed.
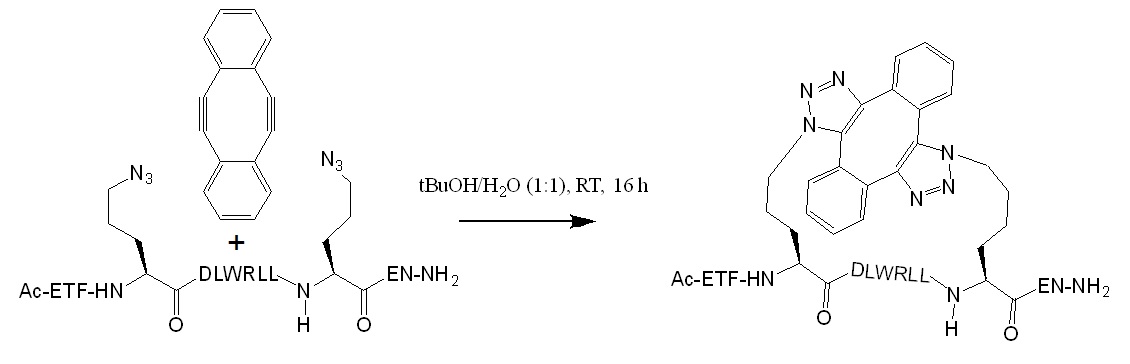
Figure 3: Double-SPAAC stabling reaction for the synthesis of a p53-derived diazidopeptide accroding to Lau et al. 2015.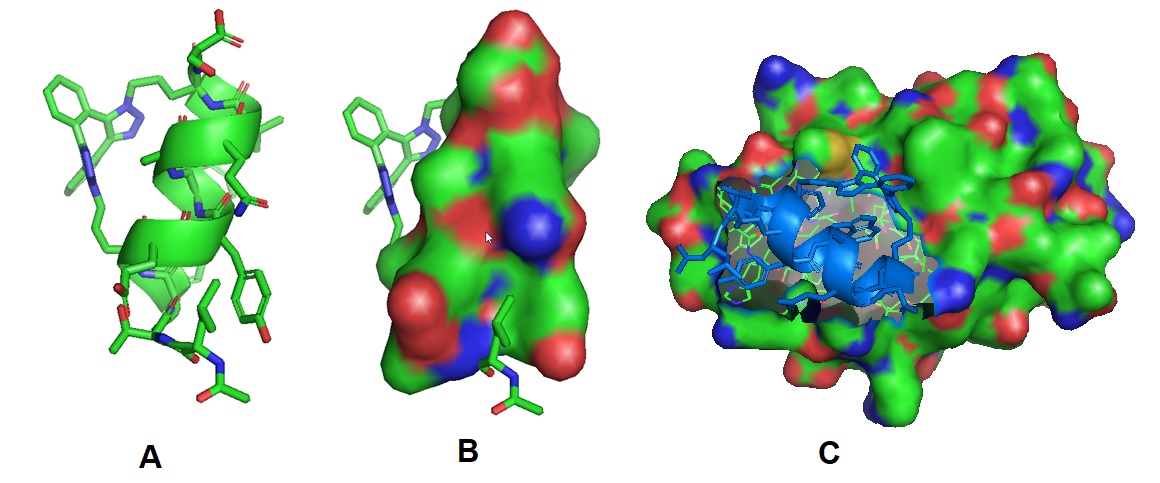
Figure 4: Structural models of the stabled peptide (A dn B) and the model when bound to MDM2.
Reference
Lau YH, Wu Y, Rossmann M, Tan BX, de Andrade P, Tan YS, Verma C, McKenzie GJ, Venkitaraman AR, Hyvönen M, Spring DR. Double Strain-Promoted Macrocyclization for the Rapid Selection of Cell-Active Stapled Peptides. Angew Chem Int Ed Engl. 2015 Dec 14;54(51):15410-3. doi: 10.1002/anie.201508416. Epub 2015 Nov 2. PMID: 26768531; [PMCID: PMC5868729].
Mitsuru Miyachi, Naoki Kakazu, Shigeki Yagyu, Yoshiki Katsumi, Satoko Tsubai-Shimizu, Ken Kikuchi, Kunihiko Tsuchiya, Tomoko Iehara and Hajime Hosoi; Restoration of p53 Pathway by Nutlin-3 Induces Cell Cycle Arrest and Apoptosis in Human Rhabdomyosarcoma Cells. Cancer Therapy: Preclinical. DOI: 10.1158/1078-0432.CCR-08-2955 Published June 2009. [Pubmed]
Wang S, Zhao Y, Aguilar A, Bernard D, Yang CY. Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapy: Progress and Challenges. Cold Spring Harb Perspect Med. 2017 May 1;7(5):a026245. doi: 10.1101/cshperspect.a026245. PMID: 28270530; [PMCID: PMC5411684].
---...---