The successful development of synthetic chemistry methodology allowed the production of high-quality oligonucleotides that are cost effective. The availability of the synthetic oligonucleotides opened up multiple opportunities for novel basic research, diagnostic and therapeutic applications. With increasing utility, came the demand for further modification to enhance oligonucleotide’s binding affinity to various targets including DNA, RNA and protein. Equally significant is the chemical modification to block degradation by nucleases in vivo. For clinical application, oligonucleotide drugs must meet additional criteria including delivery, potency, toxicity, off-target activity, etc.
Xeno nucleic acids collectively refer to nucleic acid analogues that have been chemically modified. Among them is locked nucleic acid (LNA), representing modified RNA nucleotide with an extra bridge linking 2' oxygen and 4' carbon of the sugar moiety. By locking the ribose in the 3'-endo conformation, the bridge reduces the entropic cost for transitioning from single stranded state to A-form helix (Julien et al., 2008). This entropic advantage translates into significantly higher binding affinity to target complementary strand, promoting hybridization and augmenting Tm. Hence, a significant increase in the stability of duplex (RNA:DNA, DNA:DNA) or triplex can be achieved by incorporating even a smaller number of LNAs.
This, in turn, inspired the development of 2'-O,4'-aminoethylene bridged nucleic acid (2',4'-BNANC), the third-generation bridged nucleic acid containing a six-member bridged structure with an N-O linkage. It was developed along the same general principle to enhance the binding affinity. The 2',4'-BNANC modification provides greater binding affinity, better single-mismatch discrimination, enhanced RNA specificity, stronger/selective triplex-forming properties, and considerably higher nuclease resistance (Rahman et al., 2008; Miyashita et al., 2007). Antisense oligonucleotides containing 2',4'-BNANC are more stable and better tolerated than LNA modified oligonucleotides in the murine models (Rahman et al., 2008; Yamamoto et al., 2012). Further, 2',4'-BNANC modified oliigonucleotides induce lesser caspase activity, explaining its reduced toxicity (Manning et al., 2017). These attributes make 2',4'-BNANC ideal for clinical application.
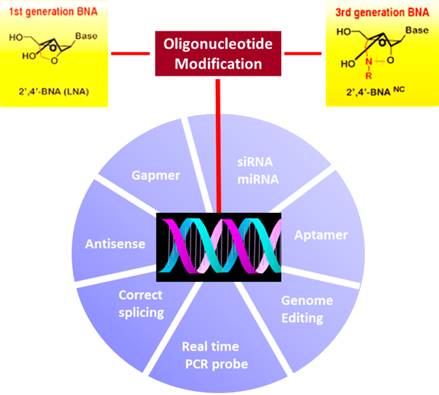
A wide range of applications are possible with conformationally restricted nucleoside analogues, which includes basic science research. For the structure-function relationship study concerning the role of ribose structure and dynamics in RNA function, LNA was used to probe the site-specific conformational/energetic properties in ribozyme (Julien et al., 2008). For genome editing, incorporating LNA or BNANC at specific points in CRISPR-RNAs (crRNAs) decreased off-target DNA cleavage by Cas9, improving specificity (Cromwell et al., 2018). The potential use of BNANC to cap aptamers using terminal transferase to increase nuclease resistance has been described (Kim et al., 2015). Other potential uses include modulating mRNA splicing by oligonucleotides with such modification.
For diagnosis, LNA or 2',4'-BNANC incorporated into oligonucleotide probes greatly improved the efficacy of SNP genotyping, allelic discrimination, real time PCR amplification, qPCR, hybridization probe or FISH analysis. As LNA bases render the probe greater duplex stability than single MGB (minor grove binders) at the 3’ end, it provides an ideal substitute for TaqMan MGB probes. Using bead-based suspension assay with BNANC modified hybridization probes, specific mutation in DNMT3A gene was quantified in hematological malignancies, for which BNANC probes performed better than LNA probes (Shivarov et al., 2014). For detecting single T790M mutation in EGFR, Bio-Synthesis, Inc. developed a highly effective method using 2',4'-BNANC modified clamp for real-time PCR (Kim et al., 2015).
The utility of bridged nucleic acids for therapy is increasingly being recognized. For gene knockdown, antisense oligonucleotides have been used to inhibit gene expression by triggering RNase-H mediated cleavage of targeted mRNAs or through hybrid-arrest of binding/scanning by mRNA translation complexes. These include antisense LNA oligodeoxynucleotides that bind to complementary H-Ras mRNA or miR-17-5p microRNA (miRNA) to inhibit cancer progression (Fluiter et al., 2005). The antisense oligonucleotides with BNANC targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), which is involved in LDL receptor regulation, lowered the cholesterol level (Yamamoto et al., 2012). BNANC modified antisense oligonucleotide targeting aac(6′)-Ib gene conjugated to a permeabilizing peptide increased susceptibility to the antibiotic amikacin in the pathogen Acinetobacter baumannii (Lopez et al., 2015). A gapmer incorporating BNANC efficiently degraded CUG expanded repeat RNA, which causes myotonic dystrophy (Manning et al., 2017). For RNA interference, the LNA modification endowed greater functionality/stability to siRNA therapeutics (Elmén et al., 2005).
Bio-Synthesis, Inc. specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology that we offer provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power was especially useful for diagnosis (ex. FISH using DNA probe). More importantly, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides for clinical application.
https://www.biosyn.com/oligonucleotide-modification-services.aspx
References
Cromwell CR, Sung K, Park J, Krysler AR, Jovel J, Kim SK, Hubbard BP. Incorporation of bridged nucleic acids into CRISPR RNAs improves Cas9 endonuclease specificity. (2018) Nat Commun. 9:1448. doi: 10.1038/s41467-018-03927-0.
Elmén J, Thonberg H, Ljungberg K, Frieden M, Westergaard M, Xu Y, Wahren B, Liang Z, Ørum H, Koch T, Wahlestedt C. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. (2005). Nucleic Acids Res. 33:439-47. PMID: 15653644
Fluiter K, Frieden M, Vreijling J, Rosenbohm C, De Wissel MB, Christensen SM, Koch T, Ørum H, Baas F. On the in vitro and in vivo properties of four locked nucleic acid nucleotides incorporated into an anti-H-Ras antisense oligonucleotide. (2005).Chembiochem. 6:1104-9. PMID: 4244378 PMCID: PMC3824000 DOI: 10.1371/journal.pone.0078863
Julien KR, Sumita M, Chen PH, Laird-Offringa IA, Hoogstraten CG. Conformationally restricted nucleotides as a probe of structure-function relationships in RNA. (2008) RNA. 14:1632-43. PMID: 18596252 doi: 10.1261/rna.866408.
Kim S-K, Liu X, Castro A, Loredo L, Castro M. An effective detection method for the EGFR single mutation T790M using BNA-NC clamping real-time PCR. (2015) Proc Am Assoc Cancer Res 56:1197–1198.
Lopez C, Arivett BA, Actis LA, Tolmasky ME. Inhibition of AAC(6')-Ib-mediated resistance to amikacin in Acinetobacter baumannii by an antisense peptide-conjugated 2',4'-bridged nucleic acid-NC-DNA hybrid oligomer. (2015) Antimicrob Agents Chemother. 59:5798-803 doi: 10.1128/AAC.01304-15.
Manning KS, Rao AN, Castro M, Cooper TA. BNANC gapmers revert splicing and reduce RNA foci with low toxicity in myotonic dystrophy cells. (2017). ACS Chem Biol. 12:2503-2509. PMID: 28853853 PMCID: PMC5694563 doi: 10.1021/acschembio.7b00416.
Miyashita, K., Rahman, S. M., Seki, S., Obika, S., and Imanishi, T. N-Methyl substituted 2′,4′- BNANC: a highly nuclease resistant nucleic acid analogue with high-affinity RNA selective hybridization. (2007) Chem. Commun. (Cambridge, U. K.), 3765−3767.
Rahman SM, Seki S, Obika S, Yoshikawa H, Miyashita K, Imanishi T. Design, synthesis, and properties of 2',4'-BNA(NC): a bridged nucleic acid analogue. (2008) J Am Chem Soc. 130:4886-96. PMID: 18341342 doi: 10.1021/ja710342q.
Shivarov V, Ivanova M, Naumova E. Rapid detection of DNMT3A R882 mutations in hematologic malignancies using a novel bead-based suspension assay with BNA(NC) probes. (2014) PLoS One. 9:e99769. doi: 10.1371/journal.pone.0099769.
Yamamoto, T., Harada-Shiba, M., Nakatani, M., Wada, S., Yasuhara, H., Narukawa, K, et al. Cholesterol-lowering action of BNA-based antisense Ooonucleotides targeting PCSK9 in atherogenic diet-induced hypercholesterolemic mice. (2012) Mol. Ther.– Nucleic Acids 1, e22.