Bioconjugate Chemistry for Molecular Engineering
A "bioconjugate" refers to a molecular species that can be produced by living systems or synthetically by chemists or biologists. A bioconjugate is composed of at least two different molecular parts of biological origin. Bioconjugates used in organisms or cells are designed such that they are water soluble or that cells are able to compartmentalize them. For synthetic production of
Bioconjugate chemistry, often also called bioconjugation chemistry, is a research field that studies the linking of one molecule to another by chemical or biological means. Typically, the resulting complexes are formed from at least one biomolecule, however, several molecules may be conjugated together as well. In addition, purely synthetic conjugated molecules are possible as well.
The study and use of chemical conjugation reactions, now also known as bioconjugation reactions, has more recently evolved into an important research topic as can be monitored by the number of publications in this field. For example, site-specific bioconjugations of a multitude of biomolecules to proteins, DNA, RNA, and carbohydrates, or to each other, have been developed. The resulting conjugates are useful for applications such as ligand discovery, disease diagnosis, and high-throughput screening, in vivo imaging, sensing, catalysis, therapeutics, as well as cell targeting. More recently, polymer brushes linked with biotin moieties allowing for the development of streptavidin-mediated conjugation capture agents in NanoVelcro chips have been engineered. These conjugates are a new type of molecular probes for prenatal diagnostics (GEN July 2015).
General bioconjugation chemistry schemes are illustrated in figure 1. Typically, bioconjugation reactions are employed to couple biomolecules to surfaces or solid supports. Typical supports are beads, gold surfaces, nitrocellulose or
Image may be NSFW.
Clik here to view.
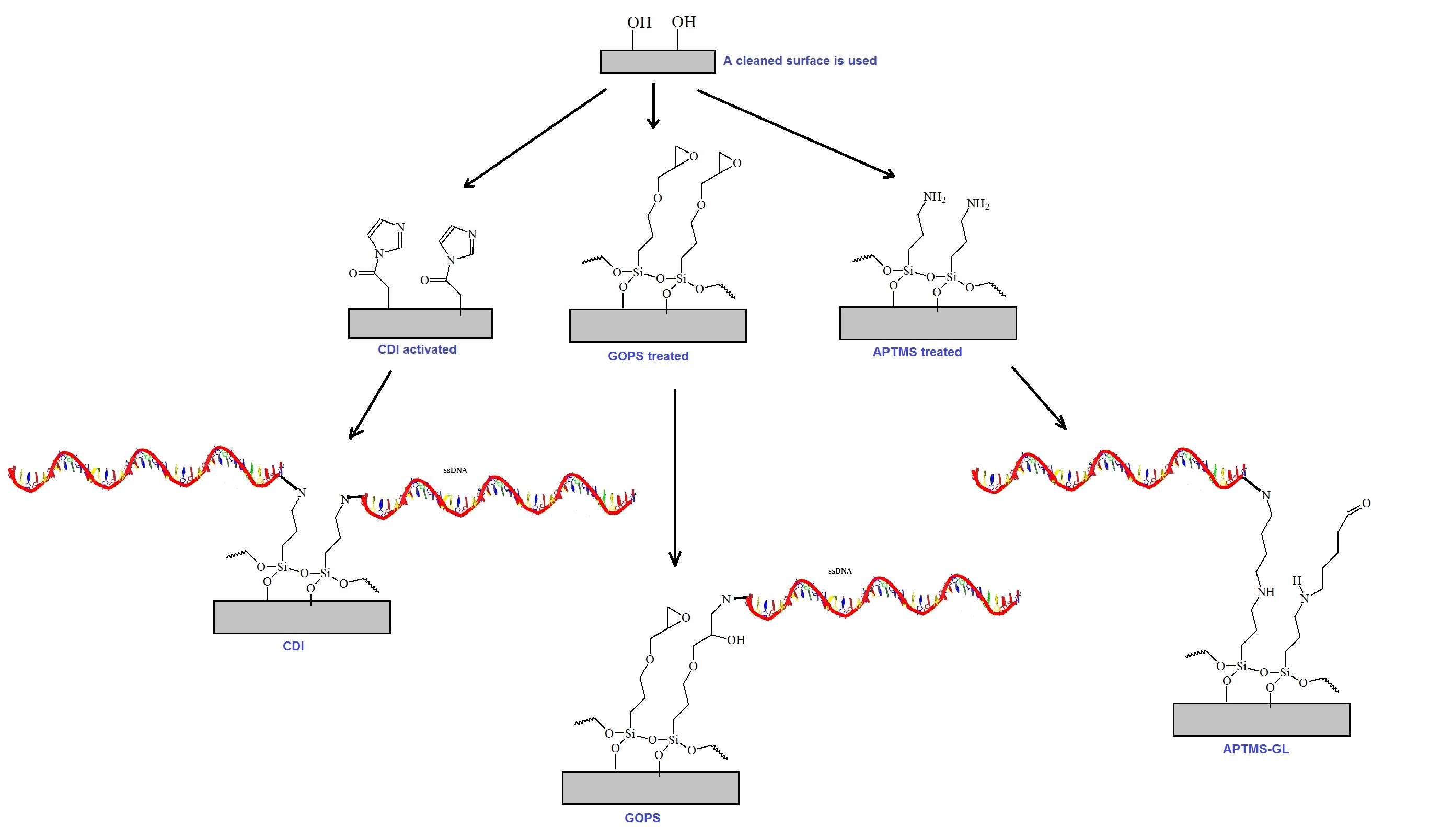
Figure 1: General schematic of bioconjugation chemistries. The conjugation of oligonucleotides to a solid support or surface is illustrated here. Three bioconjugation chemistries commonly used are reviewed. Carbonyl-diimidazole (CDI) activation to silanol groups is shown to the left. The surface functionalization technique using
Bioengineering or Biological engineering is a new scientific field that applies engineering principles to biological systems. Broadly viewed, bioengineering can include elements of electrical, mechanical, computer science, materials, chemistry, biology and medical biology. Its main goal is to apply concepts and methods observed in biological systems to solve real-world problems in life sciences. Often medical biology is part of this endeavor. Bioengineering uses primarily knowledge gained from the fast developing field called “molecular biology.” A rapid development of many innovative techniques and methodologies pertinent to biological or medical applications using bioengineering principles leading to new applications in medicine, agriculture, and energy or electronic production occurred in recent decades. A new branch known as “biomimetics” strives to engineer new materials to mimic structures and functions of molecules found in living organisms using DNA, RNA, and protein molecules. The goal is the production or manufacture of new types of nano-materials, such as hydrogels,
(1) Formation of amide bonds, including urea and thiourea moieties.
Typically amide bonds are formed through the reaction of an amino group with a carboxylic acid. Activator reagents are utilized to form the bonds more efficiently. Activator molecules such as 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC),
Figure 2. Amide formation reactions frequently used in bioconjugation.
(2) Formation of thioethers
The formation of thioesters is a widely used conjugation chemistry known for quite some time now. Reactions are very selective and specific since thiol groups are available in proteins that contain cysteine. Some proteins, such as members of the C3/α-2M thioester protein family, and peptides that contain lanthionine, contain natural interchain thioester bonds. The formation of thiols bonds in biomolecules is relative straight forward. Also, incorporation of thiol groups into synthetic oligonucleotides and peptides is done with the help of standard chemistries. An advantage of the thioester chemistry is that primary amines do not interfere with the reaction avoiding the formation of byproducts. Also, reactions can be performed at a broad pH range, ranging from pH 2 to 10. Therefore, many hetero-bifunctional cross-linkers are typically designed to contain one thiol-reactive group
Figure 3. Frequently used bioconjugation thioether formation reactions.
(3) Conjugation reactions involving carbonyl group
The presence of carbonyl groups in biomolecules is very limited. However, these are important functional groups allowing the conjugation of saccharide moieties. Except for the aldehyde and ketone groups of the linear saccharides, hydroxyl groups at the anomeric position of the cyclic saccharides normally are not reactive enough and, therefore, are not available for bioconjugation reactions. In particular, 1,2-diol groups in saccharides can be specifically oxidized to aldehydes with the help of sodium periodate. Carbonyl groups can be introduced to biomolecules using cross-linkers such as N-succinimidyl-4-formylbenzamide (S-4FB). Carbonyl groups are reactive towards primary amines, including hydrazine and
Figure 4. Conjugation reactions involving carbonyl groups.
(4) Thiol-exchange reactions
Thiol-exchange reactions are widely used because these reactions produce cleavable conjugates. Disulfide bonds are created between the conjugated molecules. Disulfide bonds containing conjugates can be cleaved via reduction by thiol reductases in tissue or cells. Furthermore, disulfide bonds can also be chemically cleaved in vitro using reducing agents such as mercaptoethanol (2-ME), tris(2-carboethyl)phosphine (TCEP), or dithiothreitol (DTT). However, the reactions for the formation of conjugates are usually slow but very selective towards the thiolated molecules. Amino or hydroxyl groups don’t usually interfere to produce byproducts. Although sometimes methanethiosulfonate reagents are used, 2-pyridyldithiol reagents are the most widely used
Figure 5. The
(5) Click chemistry and tetrazine ligation
Click chemistry has gained a huge momentum in bioconjugation
Figure 6. Click chemistry (A) and tetrazine ligation (B)
(6) Photoreactive cross-linkers
Photoreactions are only frequently performed in bioconjugation reactions. However, they are occasionally used for in-vivo or in-vitro crosslinking reactions. Photoreactive cross-linkers commonly used are
Figure 7.Photoreactions frequently used in bioconjugation.
References
Advances in Bioconjugation: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2901115/
BC Bioconjugate Chemistry Journal: http://pubs.acs.org/journal/bcches
Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy