Complexity of the modern synthetic biology dictates attachment and labeling methods that would not interfere with parallel biological process. Conventional methods are very limited by their chemistry, therefore it is important to have arsenal of completely orthogonal and chemo-selective methods. That is why during last decade dozens of new attachment and labeling methods have been developed that increased availability and versatility of the conjugation tools.
Carboxyl modified oligonucleotides
Oligonucleotide conjugation is predominantly carried out by use of a nucleophilic group on an oligonucleotide to react with an electrophilic group on a reporter molecule or a solid support. This is predominant approach because the common oligonucleotide deprotection is performed by base treatment e.g. ammonia, primary alkylamines or their combinations which are inherently nucleophilic. However, there are many situations when researchers need to introduce an electrophilic group into oligonucleotides and use it in the attachment method towards of a nucleophilic moiety.
In the case, when oligonucleotides have been used in that type of the bio-conjugation the activated carboxylate have been generated post-synthetically.2 Free carboxyl modified oligonucleotides can be activated by EDC in situ in the organic or aqueous conditions and subsequently conjugated to aminated counterpart (Fig. 1). However, in order to generate free carboxylate attached oligonucleotide using commercially available building blocks it requires cleavage of the ester bond with alkaline base4,5 before oligonucleotide base deprotection in concentrated ammonia, otherwise the ester will beconverted into corresponding amide.
Figure 1. Bio-conjugation between carboxyl modified oligonucleotide and alkylamino moiety via EDC reagent.
Huisgen’s 1-3 Dipolar cycloaddition
The Copper (I) catalyzed Huisgen’s 1,3-dipolar cycloaddition between alkynes and azides discovered by the Sharpless group in 2002 (“click chemistry”),9 is a novel and very potent method for incorporation of molecules of interest (reporter molecules, lipophilic ligands, etc.) into oligonucleotides. The methods have been limited to the post-synthetic attachment of labels, and the proposed methods have not been commercially viable alternatives to standard synthesis approaches.10-12
Recently Prof. Brown’s group discovered that the neutral heteroaromatic “click” backbone, when it introduced instead of natural phosphodiester bond is acceptable for Taq polymerase and can be used for most polymerase dependent proceses.13
Cooper dependent “click chemistry” often limits that type of attachment chemistry due to cytotoxicity. Recently developed azadibenzocyclooctyne doesn’t require any catalysts and it is highly reactive towards aliphatic and aromatic azides.14 
Diels-Alder attachment method
Another important catalyst free chemo-selective attachment method is Diels-Alder reaction that was successfully employed in bio-conjugation.15 In order to make this process highly efficient at ambient temperature, the alkyldienyl group should be activated with electron donating group (EDG) and the dienophile should have adjacent electron withdrawing group (EWG).
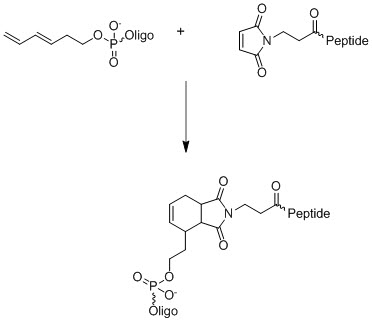
Figure 4. Diels-Alder reaction used in bio-conjugation.
Bio-synthesis offers not only wide varieties modified oligonucleotides and also their conjugates with peptides, proteins and antibodies.1
References:
- http://www.biosyn.com/Bioconjugation.aspx
- E. Jablonski, E. W.Moomaw, R. H.Tullis and J. L.Ruth Nucleic Acid Res., 1986, 14, 6115-6128.
- J. D. Kahl and M. M. Greenberg J. Org. Chem., 1999, 64 (2), 507–510
- A.V. Kachalova, T. S. Zatsepin, E. A. Romanova, D. A. Strelenko, M. J. Gait, T. S. Oretskaya Nucleosides, Nucleotides Nucleic Acids. 2000, 19, 1693-1707
- T. P. Prakash, A. M. Kawasaki, E. A. Lesnik, S. R. Owens, M. Manoharan Org. Lett. 2003, 5, 403-406.
- M. A. Podyminogin, E. A. Lukhtanov and M. W. Reed Nucleic Acid Res., 2001, 29, 5090-5098.
- S. Raddatz, J. Mueller-Ibeler, J. Kluge, L. Wäß, G. Burdinski, J. R. Havens, T. J. Onofrey, D. Wang, and M Schweitzer Nucleic Acid Res., 2002, 30, 4793-4802.
- E. N. Timofeev, A. D. Mirzabekov, S. V. Kochetkova and V. L. Florentiev Nucleic Acid Res., 1996, 24, 3142-3148.
- V. V. Rostovtsev, L.G. Green, V. V. Fokin, K.B. Sharpless, Agnew. Chem. Int. Ed., 2002, 41, 2596-2599.
- A.V. Ustinov, et al, Tetrahedron, 2007, 64, 1467-1473.
- Agnew, B. et al., US Patent application 20080050731/A1.
- X. Ming, P. Leonard, D. Heindle and F. Seela, Nucleic Acid Symposium Series No. 52, 471-472, 2008.
- A. H. El-Saghner, and T. Brown, Accounts of Chemical Research, 2012, 45 (8), 1258-67.
- M. F. Debets, S. S. van Berkel, S. Schoffelen, F. P. J. T. Rutjes, J. C. M. van Hest and F. L. van Delft, Chem. Commun., 2010,46, 97-99.
- V. Marcha ´n, S. Ortega, D. Pulido, E. Pedroso and A. Grandas, Nucleic Acid Res., 2006, 34, e24