Likewise, the incidence rate of the neurodegenerative disorder Alzheimer's disease has also been rising and is expected to affect 131 million individuals by 2050 worldwide (47 million affected currently) (Jaul et al., 2017). In the U.S., Alzheimer's disease (6th leading cause of death) affects ~5.8 million individuals and is expected to reach 14 million by 2050. The highest incidence rates were reported in Western Europe or North America, followed by Latin America, China, and Western Pacific. It affects principally those over 75-85 years of age and the risk factors include diet (dyslipedimia, elevation of lipid level in the blood), obesity, diabetes, and reduced physical activity. The principal symptom associated with Alzheimer's disease is dementia (loss of memory) and cognitive impairment; as such, engaging in intellectual activities may lessen the risk. Currently, there is no cure for Alzheimer's disease.
Though the underlying cause remains unknown, several mechanisms have been proposed. As with cancer, Alzheimer's disease can be classified as early-onset or late-onset type. Whereas the late-onset cases are sporadic, some of the early-onset cases (occurring before 65 y) could be hereditary. Genetic studies of the familial cases (symptoms occurring in 40's-50's) have identified several predisposing genes, which include APP (chromosome 21), PSEN1 (chromosome 14) and PSEN2 (chromosome 1). They encode proteins that affect the processing 'amyloid precursor protein' (APP).
One hypothesis suggests that the build-up of amyloid-beta plaques outside the neurons may cause chronic inflammation (when immune cells could no longer clear the toxins), causing the death of neuronal cells. The plaques are generated when the cell membrane-bound APP protein is cleaved at several points along the polypeptide, liberating amyloid-beta peptide (ABP), which then aggregates to form oligomers (which, in turn, form fibrils) within the brain. One of the enzymes involved in the proteolytic cleavage of APP is gamma-secretase, which is comprised of 4 subunits including presenilin-1 (encoded by PSEN-1 gene) (Chen et al., 2019).
Another gene implicated in Alzheimer's disease is ApoE gene, which is involved in trafficking lipids within the brain, and may facilitate the degradation of amyloid-beta peptide (Jiang et al., 2008). The other contributing factor to Alzheimer's disease is the accumulation of Tau protein (microtubule associated protein) within the neuronal cells (Tiwari et al., 2019).
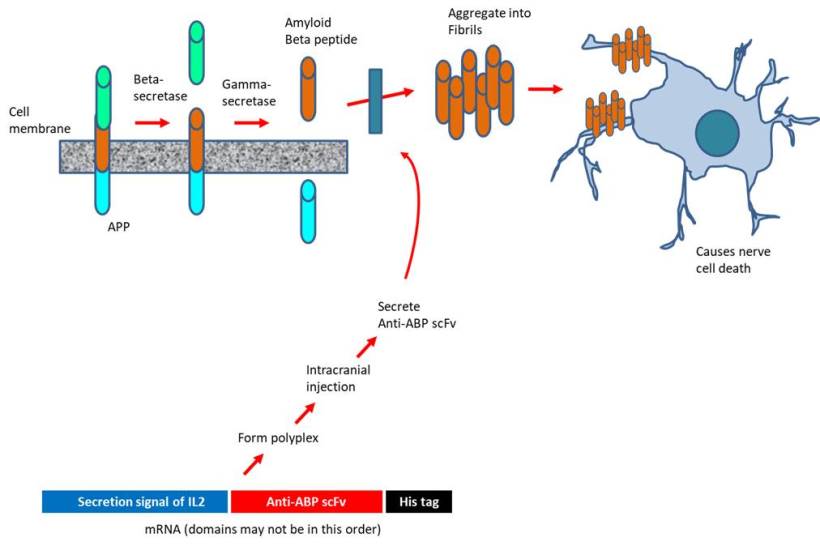
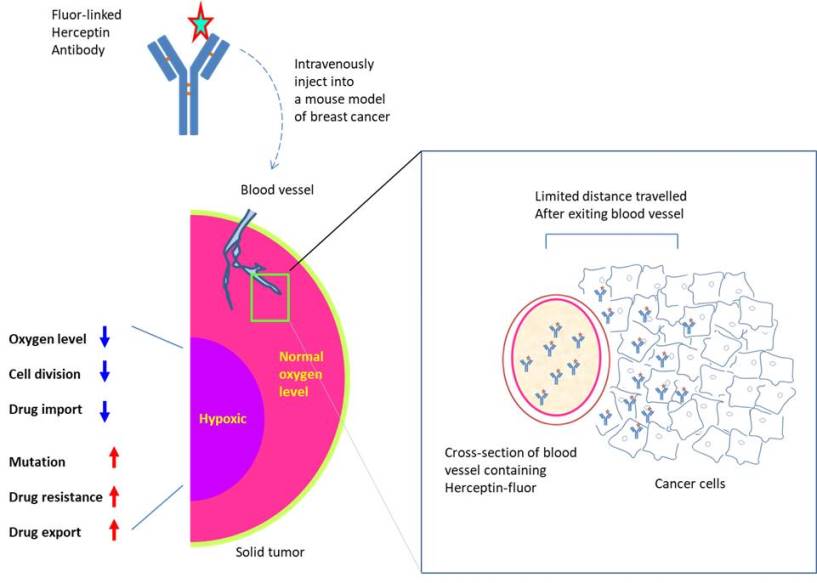
An innovative way exploiting the latest advances in mRNA technology to treat Alzheimer's disease is proposed. In the past, pharmaceutical industries have focused on injecting high doses of antibodies targeting amyloid-beta peptide or administering recombinant adenoviruses to express such antibodies. The approach is marred by the extremely low distribution to the brain (less than 1% of the systemically injected) or the side effects associated with drugs inhibiting the amyloid beta pathway. To address, the investigators at the University of Tokyo (Japan) designed mRNA encoding 3 distinct scFv (single-chain variable fragment that bind to amyloid-beta peptide) to be secreted by the expressing cells. The scFV is comprised of the variable regions of the light and heavy chains of immunoglobulins connected through a linker. The mRNA construct was delivered as polyplexes formed with copolymer PEG-PAsp (DET), i.e polyethylene glycol-poly[N'-[N-(2-aminoethyl)-2-aminoethyl] aspartamide] (Perche et al., 2017). Intriguingly, scFv expression occurred following the transfection with mRNA (but not DNA) in neurons. Upon intracranial injection, the scFv was able to reduce the level of amyloid beta peptide by 40% in an acute amyloidosis model although no such decline was observed in a transgenic model of Alzheimer's disease. Nevertheless, the authors suggest that the drug design may be applicable for the immunological treatment of other neurological disorders.
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophoreseither terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/tew/Messenger-RNA-(mRNA)-for-Vaccine-Development-Against-Coronavirus.aspx
https://www.biosyn.com/mrna.aspx
References
Chen XQ, Mobley WC. Alzheimer Disease Pathogenesis: Insights From Molecular and Cellular Biology Studies of Oligomeric Aβ and Tau Species. Front Neurosci. 13:659 (2019). PMID: 31293377
Jaul E, Barron J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front Public Health. 5:335 (2017). PMID: 29312916
Jiang Q, Lee CY, Mandrekar S, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 58:681-93 (2008). PMID: 18549781
Perche F, Uchida S, et al. Improved Brain Expression of Anti-Amyloid β scFv by Complexation of mRNA Including a Secretion Sequence with PEG-based Block Catiomer. Curr Alzheimer Res. 14:295-302 (2017). PMID: 27829339
Tiwari S, Atluri V, et al. Alzheimer's disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine. 14:5541-5554. (2019). PMID: 31410002
Yan S, Gan Y, et al. Association between refrigerator use and the risk of gastric cancer: A systematic review and meta-analysis of observational studies. PLoS One 13:e0203120. (2018) PMID: 30161245




.jpg)
.jpg)
















.jpg)
.jpg)
.jpg)