Can cancer cells or their microenvironment be targeted selectively to treat tumors?
Yes, is appears that this is possible.
A number of peptides have been reported to specifically target tumor and tumor associated microenvironments, such as the tumor vasculature, after their systematic delivery. These peptides are known as “tumor-specific internalizing peptides” (TSIPs) or “tumor homing peptides” (THPs).
Similar to anti-cancer peptides tumor-specific internalizing peptides are usually short peptides in sequence lengths of 3 to 15 amino acids that specifically recognize and bind to tumor cells or tumor vasculature. Since 1998 a number of these peptides have been identified using in vitro and in vivo phage display technology. Phage display is a molecular biology technology in which proteins or peptides are displayed on the surface of a phage as a fusion with one of the phage coated proteins. Phage display has been used intensively for the screening for protein-protein interactions. This screening method allowed for the identification of tumor-specific or tumor homing peptides that target specific tumor cells or tumor vasculature.
According to the International Agency for Research on Cancer, an agency of the World Health Organization, cancer is now the world’s biggest killer. The “World Cancer Report” showed that there were 8.2 million deaths from cancer in 2012 and predicts that cancer cases worldwide will rise by 75 % over the next two decades. By then it is estimated that up to 25 million people may be suffering from cancer worldwide. Unfortunately, despite progress made in our understanding of the molecular basis of cancer and improvements made in treatment options, mortality rate is still high. This suggests that the availability of new types, more selective drugs that fight cancer would be of great benefit to humans.
Tumor-specific internalizing peptides or tumor homing peptides have common sequence motifs like RGD, or NGR, which specifically bind to a surface molecule on tumor cells or tumor vasculature. The best known examples are the short peptides RGD and NGR. The RGD (Arg-Gly-Asp) peptide is known to bind α integrins and NGR (Asn-Gly-Arg) is known to bind to a receptor aminopeptidase N present on the surface of tumor endothelial cells, also called tumor angiogenic markers. It is no wonder that tumor-specific internalizing peptides are being used in cancer diagnosis and treatment. So far, many anti-cancer and imaging agents have been targeted to tumor sites in mice models by conjugation them to tumor-specific peptides. A database called “TumorHoPe” provides comprehensive information about experimentally validated tumor homing peptides and their target cells (http://crdd.osdd.net/raghava/tumorhope/). This is a manually curated database containing 744 entries of experimentally characterized tumor homing peptides that recognize tumor tissues and tumor associated micro environment, including tumor metastasis.
A list of some tumor homing peptide motifs
Motif | Action |
NGR (Asn-Gly-Arg) | Binds aminopeptidase N |
GSL (Gly-Ser-Leu) | Inhibition of tumor homing |
RGD (Arg-Gly-Asp) | Binds selectively to integrins which are overexpressed on endothelial cell surface in the cancer and facilitate cancer cell migration |
TSPLNIHGQKL | Hn-1 appears to be HNSCC specific. Targeted drug delivery into solid tumors. |
Models of tumor homing peptides
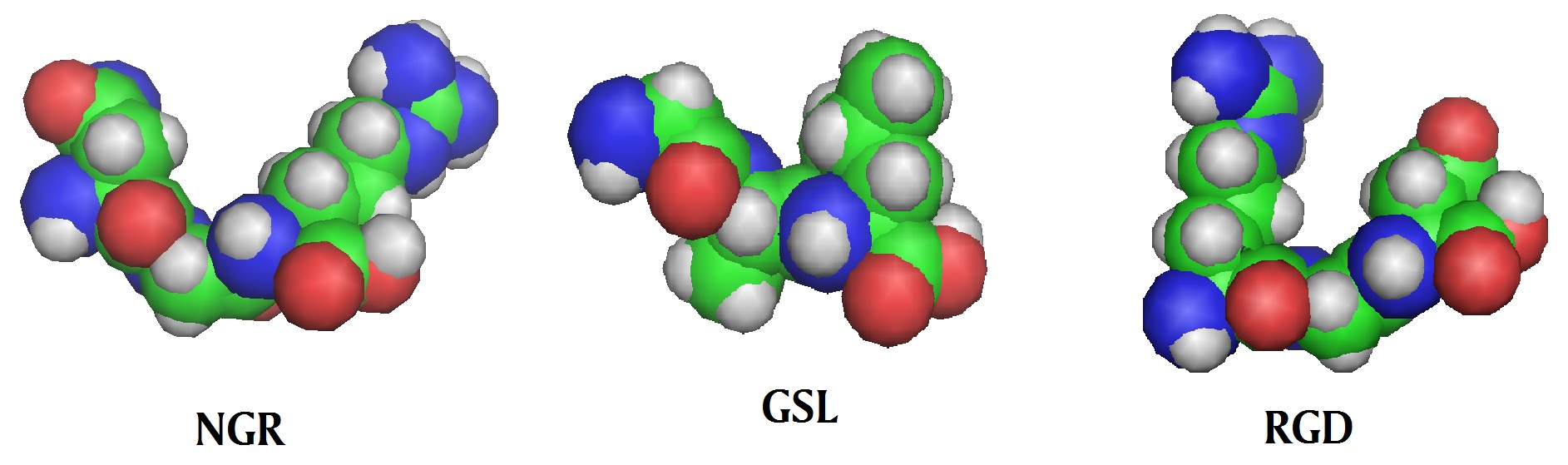
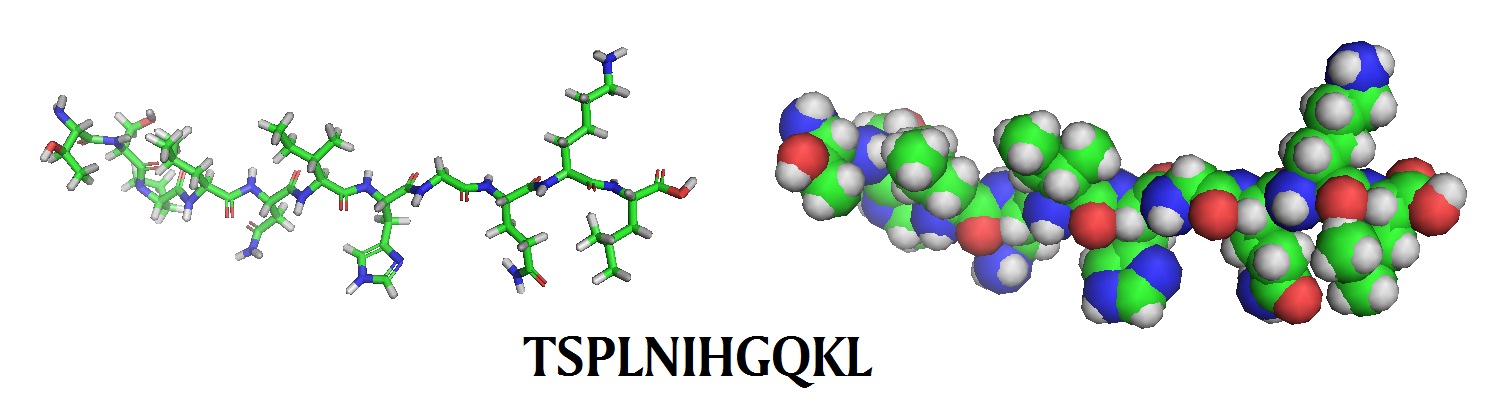
.jpg)
The specific internalization of peptides that target tumor cells has been evaluated for targeted siRNA delivery into human cancer cells. Un et al. in 2012 investigated the internalization of the HN-1TYR-anti-hRRM2 siRNAR peptide conjugate in human head and neck or breast cancer cells to establish its utility for targeted siRNA delivery into human cancer cells. The researchers used a FITC-HN-1TYR-anti-hRRM2 siRNAR construct to image its successful internalization into a human cancer cell line. For the synthesis of the fluorescent siRNA delivery vehicle, FITC-HN-1TYR-anti-hRRM2 siRNAR, a tyrosine and a FITC was added to the N-terminal end. Next, a synthetic anti-hRRM2 siRNA was synthesized with fluorine, incorporated at its 2’-OH position, to avoid degradation by RNases in vivo, and conjugated to the 5’-end of the antisense strand using a hexynyl phophoramidite linker. The selected HN1 peptide, a 12mer peptide that was isolated by peptide display library screening using a M13 phage library, contains the sequence TSPLNIHNGQKL. It has the ability to translocate drugs across the cell membrane into the cytosol, its uptake occurs in a tumor-specific manner, and it is capable of penetrating solid tumors. Ribonucleotide Reductase (RR), composed of the subunits hRRM1 and hRRM2, catalyses the conversion of ribonucleotides to their corresponding deoxy forms need for DNA replication. The researchers choose an anti-hRRM2 siRNA to allow for the degradation of hRRM2’s mRNA to suppress tumorgenesis.
To conclude, tumor-specific internalizing peptides or tumor homing peptides appear to be future drug candidates for targeted siRNA delivery into human cancer cells that may enable a more selective treatment of tumors with less site effects.
References
Kapoor P, Singh H, Gautam A, Chaudhary K, Kumar R, et al. (2012); TumorHoPe: A Database of Tumor Homing Peptides. PLoS ONE 7(4): e35187. doi:10.1371/journal.pone.0035187.
FRANK UN, BINGSEN ZHOU and YUN YEN; The Utility of Tumor-specifically Internalizing Peptides for Targeted siRNA Delivery into Human Solid Tumors. ANTICANCER RESEARCH 32: 4685-4690 (2012).