| Spectra of available Fluorescent Dyes | |||||||||
| Fluorescent Dye | Abs | Em | Labelling Position | Purification Option | Alternative to | ||||
| 5' | Int | 3' | HPSF | HPLC | PAGE | ||||
| Alexa Fluor 350 [Alexa350] | 346 | 442 | x | x | x | x | AMCA, DyLight350, iFluor350, CF350, | ||
| ATTO 390 | 390 | 476 | x | x | x | x | |||
| Dy-415 [DY415] | 418 | 467 | x | x | x | x | Coumarin (DEAC) | ||
| ATTO 425 [ATTO425] | 436 | 484 | x | x | x | x | Alexa Fluor 425 | ||
| ATTO 465 [ATTO465] | 453 | 508 | x | x | x | x | LC 480 Cyan 500 | ||
| Bodipy FL [BOFL] | 504 | 510 | x | x | x | x | |||
| Alexa Fluor 488 [Alexa488] | 495 | 519 | x | x | x | x | CY2 DY-495ATTO 495 | ||
| FAM [FAM] | 495 | 520 | x | x | x | x | CY2 DY-495ATTO 495 | ||
| Fluorescein isothiocyanate [FITC] | 495 | 520 | x | x | x | x | CY2 DY-495ATTO 495 | ||
| Fluorescein [FLU] | 495 | 520 | x | x | DY-495 ATTO 495 | ||||
| Fluorescein-dT [FLUdT] | 494 | 522 | x | x | DY-495 ATTO 495 | ||||
| ATTO 488 [ATTO488] | 501 | 523 | x | x | x | x | Cy2 | ||
| Oregon Green 488 [OG488] | 496 | 524 | x | x | x | x | Cy2 | ||
| Oregon Green 514 [OG514] | 506 | 526 | x | x | x | x | Cy2 | ||
| Rhodamine Green [RGR] | 503 | 528 | x | x | x | x | Cy2 | ||
| TET [TET] | 521 | 536 | x | x | x | x | CAL Fluor Gold 540 | ||
| ATTO 520 [ATTO520] | 516 | 538 | x | x | x | x | CAL Fluor Gold 540 Rhodamine 6G | ||
| JOE [JOE] | 520 | 548 | x | x | x | x | CAL Fluor Gold 540 Rhodamine 6G | ||
| Yakima Yellow [YAKYE] | 530 | 549 | x | x | VICCAL Fluor 560 | ||||
| Bodipy 530/550 [BO530] | 534 | 551 | x | x | x | x | VICCAL Fluor 560 | ||
| HEX [HEX] | 535 | 556 | x | x | x | x | VICCAL Fluor 560 SIMA | ||
| Alexa Fluor 555 [Alexa555] | 555 | 565 | x | x | x | x | Cy3TRITC & TMRAlexa Fluor 546 | ||
| Dy-549 [DY549] | 553 | 566 | x | x | x | x | Quasar 570 Dragonfly Orange | ||
| Bodipy TMR-X [BOTMRX] | 544 | 570 | x | x | x | x | Quasar 570 Dragonfly Orange | ||
| Cyanine3 [CY3] | 552 | 570 | x | x | x | Quasar 570 Dragonfly OrangeTYE 563 | |||
| ATTO 550 [ATTO550] | 554 | 576 | x | x | x | x | NEDCy3Rhodamine B & 6G | ||
| TAMRA [TAM] | 544 | 576 | x | x | x | Rhodamine 6G Rhodamine B | |||
| Rhodamine Red [RRE] | 560 | 580 | x | x | x | x | Rhodamine 6G Rhodamine B | ||
| ATTO 565 [ATTO565] | 563 | 592 | x | x | x | x | PETAlexa Fluor 594 | ||
| Fluorescent Dye | Abs | Em | Labelling Position | Purification Option | Alternative to | ||||
| 5' | Int | 3' | HPSF | HPLC | PAGE | ||||
| ROX [ROX] | 575 | 602 | x | x | x | x | Texas Red Alexa Fluor 594 | ||
| Texas Red [TxRed] | 583 | 603 | x | x | x | x | ROX™Alexa Fluor 594 | ||
| Cyanine3.5 [CY35] | 588 | 604 | x | x | Alexa Fluor 594 CAL Fluor Red 610 | ||||
| LightCycler 610 [LC610] | 590 | 610 | x | x | x | x | Alexa Fluor 594 CAL Fluor Red 610 | ||
| ATTO 594 [ATTO594] | 601 | 627 | x | x | x | x | Alexa Fluor 594 CAL Fluor Red 610 | ||
| DY-480 XL [DY480] | 500 | 630 | x | x | x | x | |||
| DY-610 [DY610] | 610 | 630 | x | x | x | x | Alexa Fluor 610 | ||
| ATTO 610 [ATTO610] | 615 | 634 | x | x | x | x | Alexa Fluor 610 | ||
| LightCycler 640 [LC640] | 625 | 640 | x | x | x | x | |||
| Bodipy 630/650 [BO630] | 625 | 640 | x | x | x | x | Alexa Fluor 633 | ||
| ATTO 633 [ATTO633] | 629 | 657 | x | x | x | x | LIZAlexa Fluor 633 | ||
| Alexa Fluor 647 [Alexa647] | 650 | 665 | x | x | x | x | Cy5 Quasar 670 | ||
| Bodipy 650/665 [BO650] | 650 | 665 | x | x | x | x | Cy5 Quasar 670 | ||
| ATTO 647N [ATTO647N] | 644 | 669 | x | x | x | x | Cy5 Quasar 670 | ||
| Cyanine5 [CY5] | 649 | 670 | x | x | x | Quasar 670TYE 655 | |||
| DY-649 [DY649] | 655 | 674 | x | x | x | x | Quasar 670 | ||
| Cyanine5.5 [CY55] | 675 | 694 | x | x | Alexa Fluor 680TYE 705 | ||||
| ATTO 680 [ATTO680] | 680 | 700 | x | x | x | x | Alexa Fluor 680Alexa Fluor 700 | ||
| DY-682 [DY682] | 690 | 709 | x | x | x | x | IRDye 700Quasar 705 | ||
| ATTO 700 [ATTO700] | 700 | 719 | x | x | x | x | IRDye 700Quasar 705 | ||
| ATTO 740 [ATTO740] | 740 | 764 | x | x | x | x | Cy7Alexa Fluor 740 | ||
| DY-782 [DY782] | 782 | 800 | x | x | x | x | IRDye 800 | ||
Spectra of available Fluorescent Dyes
Function and Application of 5’-Triphosphate Oligonucleotides
Synthetic oligonucleotide-based therapeutics, including antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and modified oligonucleotides, require high-quality products at high quantities and free of impurities.
Phosphorylated oligonucleotides such as 5′-triphosphates are necessary biochemical and therapeutic tools. The 5'-triphosphate modification is an essential nucleic acid modification found in all living organisms, participating in metabolic processes and providing energy to drive many processes in living cells.
The 5-triphosphate group in nucleotides plays a fundamental role in energy transfer, nucleic acid synthesis, transcription, signaling, and enzymatic reactions. Its presence provides chemical properties and molecular interactions required for these crucial biological processes.

5'-triphosphate oligonucleotides can act as antiviral and anticancer inhibitors, as substrates for polymerase chain reactions, and nucleic acids ligation reactions, allowing structural and mechanistic studies. Also, 5'-triphosphate oligonucleotides can stimulate an immune response and are intermediates in the enzymatic synthesis of m7G-5′-ppp capped RNAs.
Oligonucleotides containing a 5'-triphosphate group are short chains of nucleotides modified with a triphosphate group at their 5'-end. Various molecular biology and biotechnology applications utilize 5'-triphosphate oligonucleotides, particularly in nucleic acid-based therapeutics.
The 5'-triphosphate (5'-TP) group plays a crucial role in the function of nucleotides and nucleic acids. 5’-TP refers to three phosphate groups attached to the 5'-carbon of a nucleotide.
The triphosphate moiety is responsible for several essential functions associated with nucleic acids:
High Energy Activated Molecules: The presence of the triphosphate group in nucleotides, such as adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP), provides a high-energy bond. Hydrolysis of the terminal phosphate group releases energy, which cells can harness for various cellular processes, including muscle contraction, active transport of ions, DNA and RNA synthesis, and enzymatic reactions.
Nucleic Acid Synthesis: During DNA and RNA synthesis, the triphosphate group is critical for adding nucleotides to the growing nucleic acid chain. In DNA replication, for example, the 5'-TP group of a deoxyribonucleoside triphosphate (dNTP) is a substrate for DNA polymerase enzymes. The formation of a phosphodiester bond between the 5'-phosphate of the incoming dNTP and the 3'-hydroxyl group of the growing DNA chain is catalyzed by a polymerase, resulting in the elongation of the DNA strand.
RNA Transcription: Transcription requires the triphosphate group to initiate and elongate RNA chains. The RNA polymerase enzyme recognizes the triphosphate group at the 5'-end of the incoming ribonucleoside triphosphate (rNTP) and incorporates it into the growing RNA molecule during transcription. The 5'-TP group stabilizes the RNA polymerase binding and facilitates proper transcription initiation and elongation.
Signaling Molecules: Certain nucleotides with a triphosphate group, such as GTP and ATP, function as important signaling molecules within cells. GTP is a molecular switch in G-protein signaling pathways and several cellular processes, including cell growth, differentiation, and intracellular signaling cascades. In addition to its energy-carrying role, ATP acts as a signaling molecule in cell signaling, muscle contraction, and enzymatic reactions.
Enzymatic Reactions: The triphosphate group can participate in enzymatic reactions as a phosphate donor or acceptor. Kinases, for example, transfer a phosphate group from ATP to target molecules, regulating their activity. Similarly, phosphatases remove phosphate groups, modulating the function of their target molecules.
Some key features and applications of 5'-TP oligonucleotides are:
Immunostimulatory Properties: 5'-triphosphate (5'-TP) oligonucleotides can interact with pattern recognition receptors, Toll-like receptors 3 (TLR3), TLR7, TLR8, and TLR9 to activate the immune system. This activation leads to the induction of cytokines and other immune response mediators, making them useful in immunotherapy and vaccine development.
The cytosolic pattern recognition receptor RIG-I detects negative-stranded RNA viruses that do not have double-stranded RNA but contain panhandle blunt short double-stranded 5′-triphosphate RNA in their single-stranded genome. In 2009, Schlee et al. reported that synthetic single-stranded 5′-triphosphate oligoribonucleotides could not bind and activate RIG-I. However, the addition of the synthetic complementary strand resulted in optimal binding and activation of RIG-I.
Antiviral Activity: Certain 5'-TP oligos containing specific sequences of nucleotides can exert antiviral effects by targeting viral nucleic acids or proteins. They can interfere with viral replication and trigger immune responses against viral infections.
RNA Interference (RNAi): 5'-TP oligonucleotides as part of small interfering RNA (siRNA) molecules trigger RNA interference. RNAi silences or knocks down specific genes. The presence of the triphosphate group at the 5' end enhances the efficiency of siRNA uptake and subsequent gene silencing.
Gene Editing and Genome Engineering: In the gene-editing technique CRISPR-Cas9, 5'-TP oligonucleotides can guide the Cas9 nuclease to specific genomic targets. 5'-TP oligonucleotides serve as the template for repairing or modifying DNA sequences and enable precise gene editing and genome engineering. Here, 5΄-phosphate mimics stabilize the 5΄-end of the guide strand. The modification protects the guide RNA from phosphatase degradation and 5΄- to 3΄-exonucleases. This modification significantly enhances the efficacy of cholesterol-conjugated siRNAs and the duration of silencing in vivo. 5΄-(E)-vinylphosphonate stabilizes the 5΄-phosphate group, enabling systemic delivery and silencing in the kidney and heart.
Diagnostic and Therapeutic Applications: Diagnostic and therapeutic approaches utilize 5'-TP oligonucleotides as probes in the polymerase chain reaction (PCR) or in-situ hybridization (ISH).
Modification: 5'-TP oligonucleotides can be modified with various functional groups or conjugated to other molecules, for example, targeting ligands or therapeutic agents for enhanced specificity and efficacy in therapeutic applications.
Synthetic 5'-TP oligonucleotides are typically chemically synthesized and may require modifications to enhance stability, cell penetration, or target specificity, depending on the desired application.
Reference
ATP [Adenosine_triphosphate]
Bare, G. A. L., Horning, D. P. Chemical Triphosphorylation of Oligonucleotides. J. Vis. Exp. (184), e63877, doi:10.3791/63877 (2022). [jove]
Rig-I [RIG-I]
Thillier Y, Decroly E, Morvan F, Canard B, Vasseur JJ, Debart F. Synthesis of 5' cap-0 and cap-1 RNAs using solid-phase chemistry coupled with enzymatic methylation by human (guanine-N⁷)-methyl transferase. RNA. 2012 Apr;18(4):856-68. [PMC]
Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, Juranek S, Kato H, Kawai T, Poeck H, Fitzgerald KA, Takeuchi O, Akira S, Tuschl T, Latz E, Ludwig J, Hartmann G. Recognition of 5' triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009 Jul 17;31(1):25-34. [PMC]
Zlatev I, Lackey JG, Zhang L, Dell A, McRae K, Shaikh S, Duncan RG, Rajeev KG, Manoharan M. Automated parallel synthesis of 5'-triphosphate oligonucleotides and preparation of chemically modified 5'-triphosphate small interfering RNA. Bioorg Med Chem. 2013 Feb 1;21(3):722-32. [article]
---...---
" Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including 5'-triphosphate and back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides".
---...---
Highly nuclease resistance and thermodynamically stable 2′-O,4′-C-Ethylene-bridged Nucleic Acid (ENA) as a promising candidate for antisense therapy
2'-O, 4'-C-Ethylene-bridged Nucleic Acids (ENA) are promising modified nucleic acids in which ethylene is bridged at the furanose sugar ring at the 2'-O and 4'-C ends to form a less strained six-membered ring with C3'-endo conformation. ENA gapmers effectively recruited RNase H, and ENA act as splice modulators such as exon-skipping antisense oligonucleotides and antigene oligonucleotides. Also, clinical trials are currently being conducted using ENA oligonucleotide to treat Duchenne muscular dystrophy (DMD). The aim of the treatment is to skip the normal exon 45 of the dystrophin gene to prevent the occurrence of a stop codon in exon 45. This will restore the functional dystrophin. This technology is owned by Daiichi Sankyo, and they are conducting a Phase II clinical study in Japan.[1]
Background: Koizumi et al. developed the building block for bicyclo 2′-O, 4′-C-ethylene-bridged nucleic acid (ENA) and compared the properties of 2’,4’-BNA/LNA, a third-generation nucleic acid. Also, further development on synthetic methods to improve the productivity of the monomer has been reported.[2] Interestingly, apart from high thermodynamic stability, ENA showed enhanced nuclease resistance while retaining the binding affinity strength of other 2’,4’-BNA/LNA. ENA restricts the single-strand flexibility of RNA by forcing the North conformation of sugar puckering within nucleosides due to the 2′-O,4′-C-ethylene-linkage like 2’,4’-BNA/LNA. Studies have shown that 2′,4′-bridged nucleic acids can improve the ability of complementary strands to bind to duplexes. However, a recent report by J. Kawakami et al discovered that not all 2′,4′-bridged nucleic acids can lead to duplex stabilization. When comparing the properties of 2’,4’-C-bridged 2’-deoxynucleotide (CRN) with ENA, it was found that the introduction of one CRN into DNA/RNA duplexes destabilized them. [3,4]

Figure1: Structure of 2’,4’-BNA/LNA, ENA and their C3’- endo form
What makes ENA a better choice?
1. Binding affinity: ENA exhibits higher binding affinity to complementary RNA and can match that of 2′,4′-BNA/LNA (ΔTm=+3∼5 °C per modification).[5]
2. Nuclease resistance: ENAs exhibited greater nuclease resistance than natural DNA and 2′,4′-BNA/LNA. Including one ENA in oligonucleotides significantly enhances their resistance to exonucleases, surpassing that of 2′,4′-BNA/LNA.[6]
3. Thermal stability: ENA exhibits high thermal stability. A single modification of 2’O, 4’-C-ethyleneguanosines resulted in a 10-fold increase in the binding constant of the DNA/RNA duplex.[7]
4. Chimeric RNA and ENA show greater efficacy compared to phosphorothioate oligodeoxynucleotides in causing exon 19 skipping in dystrophin mRNA.[8]
5. ENA exhibits a great ability to form a triplex structure with double-stranded DNA.[6]
These results indicate that ENA is more suitable as an antisense oligonucleotide and is expected to have better antisense activity than 2′,4′-BNA/LNA. These remarkable characteristics distinguish it from other nucleic acids and render it an attractive choice for developing antisense therapies. Considering its exceptional chemical stability, ENA holds significant potential for developing oligonucleotides that are resistant to nuclease degradation, thereby enhancing their efficacy and promoting their clinical utility. The unique properties of ENA make it a compelling area of research for those involved in the field of antisense therapeutics.
References
1. M. Matsuo et al., Genes, 2017, 8, 67.
2. M. Michida et al., Organic Process Research and Development, 2022, 26, 1289-1307.
3. J. Kawakami et al., Nucleosides, Nucleotides & Nucleic acids, 2024, 1, 57-64.
4. M. Koizumi, Biological and Pharmaceutical Bulletin, 2004, 27, 453-456.
5. M. Koizumi et al., Bioorganic & Medicinal Chemistry Letters, 2002, 12, 73–76.
6. M. Koizumi et al., Bioorganic & Medicinal Chemistry, 2003, 11, 2211–2226.
7. M. Koizumi et al., Nucleic Acids Symposium Series, 2005, 49, 171-172.
8. M. Matsuo et al., Oligonucleotides, 2004, 14, 33-40.
Transfection of neurons is possible with a Steryl-R8 siRNA mixture
In the endogenous regulatory process called RNA interference (RNAi), short double-stranded RNA oligonucleotides cause sequence-specific posttranscriptional silencing of genes. Small interference RNA (siRNA) has now emerged as a promising therapeutic strategy for the development of novel drugs. However, for successful systemic delivery of siRNA therapeutics, siRNAs must penetrate cellular barriers to reach their targets in the cytoplasm.
The commonly utilized intravenous injection of siRNA poses a significant challenge. After intravenous injection, the blood circulation distributes siRNAs to organs where, at the same time, the siRNA undergoes elimination. When arriving in an organ, siRNA enters the interstitium by leaving the intravascular space within a blood vessel. From the tissue interstitium, siRNA is transported across the interstitial space to target cells. The target cell internalizes siRNA via endocytosis. This process encapsulates siRNA in endocytic vesicles that fuse with endosomes. After entering the cell, siRNA must escape from the endosomes into the cytosol for RISC loading.
Among other approaches, cell-penetrating peptides enable systemic siRNA delivery. Utilizing a cell-penetrating peptide recently, Tönges et al. developed a more straightforward method to deliver siRNA into rat neurons.
A cell-penetrating peptide modified with a stearyl moiety known as stearyl-R8 enables peptide-mediated transfection of cells and possible organelles.
|
|
Stearly-R8 (2) | siRNA (1) |
Tönges et al. 2006 showed that a stearylated octaarginine peptide (stearyl-R8) and artificial virus-like particles facilitate the transfection of siRNA into primary rat neuron cells.
The arginine peptide-siRNA complex shuttles the oligonucleotide through the cell membrane into the cell. Complexing siRNA oligonucleotides with stearyl-R8 removes the need for the covalent attachment of the siRNA to the arginine carrier peptide.
Tönges et al. found that peptide-mediated transfection with stearyl-R8 and the polymer/lipid-based cellular delivery with artificial virus-like particles are both easy to handle. Only pipet-based mixing of siRNA with the selected transfection reagent is needed. No further chemical modifications of the siRNA are required.
For an efficient transfection experiment, the research group used synthetic Stearyl-R8 to prepare the Stearyl-R8:siRNA mixture with a cation-to-anion charge ratio 2:1.
This approach may also enable specific targeting of organelles, for example, mitochondria, lysosomes, the endoplasmic reticulum (ER), and the Golgi apparatus. All these organelles are enclosed with membranes or have specialized membrane-engulfed compartments embedded within the cytoplasm. Since they all perform specific roles in the cell, their dysfunction can lead to various pathophysiologies.
Reference
Geng J, Wang J, Wang H. Emerging Landscape of Cell-Penetrating Peptide-Mediated Organelle Restoration and Replacement. ACS Pharmacol Transl Sci. 2023 Jan 16;6(2):229-244. [PMC]
Tönges, L, Lingor, P, Egle, R, Dietz, GP, Fahr, A and Bahr, M (2006). Stearylated octaarginine and artificial virus-like particles for transfection of siRNA into primary rat neurons. RNA 12: 1431-1438. [PMC]
Wang J, Lu Z, Wientjes MG, Au JL. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010 Dec;12(4):492-503. [PMC]
https://www.biosyn.com/tew/MITO-Porters-Enable-Delivery-of-Antisense-Drugs-to-Mitochondria.aspx
https://www.biosyn.com/tew/Cell-penetrating-peptides-for-the-delivery-of-siRNA-into-cells.aspx
https://www.biosyn.com/tew/cell-penetrating-or-trojan-peptides-cpps.aspx
https://www.biosyn.com/tew/cell-penetrating-peptides.aspx
---...---
" Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including 5'-triphosphate and back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides".
---...---
Nedosiran, a newly approved siRNA-based Therapeutic for Kidney Disease
The recent approval of Nedosiran (RIVFLOZATM) by the FDA was another landmark moment in the field of hyperoxaluria. Hyperoxaluria is increased hepatic oxalate production, leading to recurrent calcium oxalate kidney stones.
What are kidney stones? | Image of a kidney stone. |
Kidney stones are hard objects made up from chemicals in the urine. According to the Kidney Stone Foundation, the prevalence of kidney stones in the United States has increased from 3.8% to 8.8% in the period from the late 1970s to the late 2000s. Approximately 9% of women and 11% of men have a risk of developing kidney stones. |
|
Primary hyperoxaluria is an inherited defect of oxalate metabolism that can lead to recurrent renal stones, nephrocalcinosis, and eventually end-stage renal disease.
The small interfering RNA (siRNA) therapy for treating primary hyperoxaluria was developed by Dicerna Pharmaceuticals, now a Novo Nordisk company. In the US, Nedosiran received its first approval on 29 September 2023.
Nedosiran lowers urinary oxalate levels in children aged nine and younger than nine years and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function.
PH is a family of autosomal recessive genetic disorders characterized by excessive oxalate synthesis in the liver. Excess oxalate leads to insoluble calcium oxalate crystals in the kidneys and the development of kidney stones.
The liver enzyme lactate dehydrogenase (LDH) encoded by the LDHA gene catalyzes the conversion of glyoxylate to oxalate in liver cells. This reaction is the last step of oxalate formation in the liver. Reducing LDH by inhibiting LDHA with RNA interference therapies is a promising treatment strategy for all subtypes of PH.
Following the approval of Lumasiran, Nedosiran is the second siRNA approved for the treatment of PH1. Unlike Nedosiran, Lumasiran inhibits glycolate oxidase to decrease glyoxylate overproduction in peroxisomes.
Current synthesis technologies allow the production of short oligonucleotide complexes 21 to 27 nucleotides in size. In addition, the synthesis of therapeutic siRNAs containing specific chemical modifications is now also possible. Once synthesized, siRNAs are delivered and evaluated for their activities and drug properties in vitro and in vivo models.
The first therapeutic siRNA approved by the FDA in August 2018 was Patisiran. Patisiran is a therapeutic for the treatment of hereditary transthyretin amyloidosis. In this autosomal dominant disease, the accumulation of misfolded TTR protein throughout the body results in progressive neuropathy, cardiomyopathy, and ophthalmic disease, among other end‐organ effects.
Reference
Bhasin B, Ürekli HM, Atta MG. Primary and secondary hyperoxaluria: Understanding the enigma. World J Nephrol. 2015 May 6;4(2):235-44.[PMC]
Febina, M B. Nedosiran: The rescue from hyperoxaluria. Amrita Journal of Medicine 20(1):p 44-46, January-March 2024. [AJM]
Forbes TA, Brown BD, Lai C. Therapeutic RNA interference: A novel approach to the treatment of primary hyperoxaluria. Br J Clin Pharmacol. 2022 Jun;88(6):2525-2538. [PMC]
National Kidney Foundation [Kidney.org]
Syed YY. Nedosiran: First Approval. Drugs. 2023 Dec;83(18):1729-1733. doi: 10.1007/s40265-023-01976-4. Erratum in: Drugs. 2024 Jan 22. [PMC]
---...---
" Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including 5'-triphosphate and back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides".
---...---
A Collection of Approved Antisense Therapeutic Drugs 2024
Antisense oligonucleotides (ASOs) are short, synthetic, single-stranded oligodeoxynucleotides. ASOs can alter RNA and reduce, restore, or modify protein expression through several mechanisms. ASO-mediated therapies target the source of the pathogenesis, thereby having a higher chance of success than therapies targeting downstream pathways. An improved understanding of antisense pharmacology enabled the translation of these therapeutics into the clinic. Several ASO-mediated therapies have now received approval from the US Food and Drug Administration. However, enabling successful ASO therapies in the clinic requires optimizing ASO delivery, target engagement, and safety profiles.
Synthetic antisense oligonucleotides can modulate RNA function, influencing gene expression levels, exon skipping, and epitranscriptomics. However, understanding the function of various RNAs and the proteins they interact with can take time and effort.
The ASO technology can potentially change the therapeutic landscape for many neurological and non-neurological conditions soon. Antisense technology promises to deliver therapeutics for treating diseases by targeting RNA.
Modifications in approved oligonucleotide-based drugs are mainly based on a few sugar and backbone modifications. Modifications use in earlier ASO drugs are 2’-fluoro (2’-F), 2’-O-Methyl (2’-O-Me), phosphorothioate (PS) chemistries and 2’-O-methoxyethyl (2’-O-MOE) RNA and neutral phosphorodiamidate morpholino oligomer (PMO) backbone analogs.
Bio-Synthesis offers a comprehensive suite of technologies to enable your RNA research and help you to answer these critical questions.
Selected References
Egli, M., Manoharan, M.; Chemistry, structure and function of approved oligonucleotide therapeutics, Nucleic Acids Research, Volume 51, Issue 6, 11 April 2023, Pages 2529–2573, NAR
Rinaldi, C., Wood, M. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol 14, 9–21 (2018). Nature
Table 1: Approved Antisense Drugs
Name | Category | Approval Date | Indications |
|
|
|
|
Fomivirsen (Vitravene) 5'-GCGTTTGCTCTTCTTCTTGCG-3', Phosphorothioate Oligonucleotide | ASO | 1998.08 | Cytomegalovirus Retinitis |
Pegaptanib (Macugen) | Aptamer | 2004.12 | Age-Related Macular Degeneration |
Mipomersen (Kynamro) Phosphorothioate Oligonucleotide. Mipomersen | ASO | 2013.01 | Homozygous Familial Hypercholesterolemia |
Defibrotide (Defitelio) Deoxyribonucleic acid derivative extracted from mammalian organs. | ss-DNA and ds-DNA | 2016.03 | Hepatic Veno- Occlusive Disease |
Eteplirsen (Exondys 51) Eteplirsen is a morpholino antisense oligomer which triggers excision of exon 51 during pre-mRNA splicing of the dystrophin RNA transcript. | ASO Morpholino | 2016.09 | Duchenne Muscular Dystrophy |
Nusinersen (Spinraza) Phosphorothioate Oligonucleotide. Nusinersen | ASO | 2016.12 | Spinal Muscular Atrophy |
HepB-CpG (HEPLISAV-B) 5’-TGACTGTGAACGTTCGAGATGA-3’ HEPLISAV-B is a hepatitis B vaccine composed of recombinant hepatitis B virus surface antigen particles (rHBsAg) mixed with a synthetic oligonucleotide containing CpG motifs that stimulate innate immunity through TLR9, containing CpG oligonucleotide as adjuvant! | 22-mer PS DNA Vaccine | 2017.11 | Hepatitis B |
Patisiran (Onpattro) | siRNA | 2018.08 | Heterotrophic Transthyretin Amyloidosis |
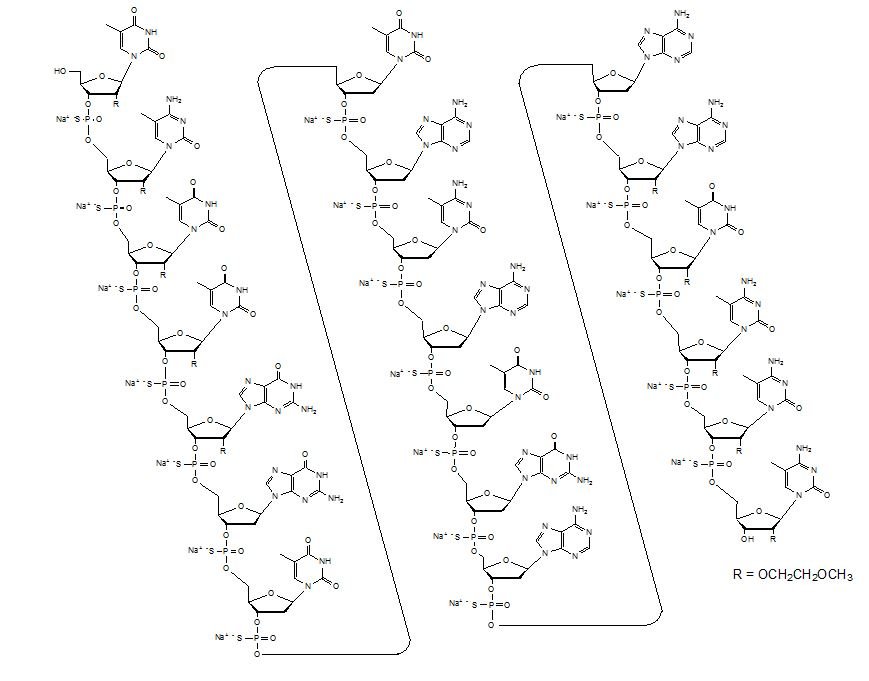
Inotersen (Tegsedi) ASO with sequence TCTTG GTTACATGAA ATCCC, where C is methylated C, and the first and third section (bases 1-5 and 16–20, separated from the middle section by spaces) are MOE-modified. | ASO | 2018.01 | Hereditary Transthyretin Amyloidosis, Polyneuropathy |
Volanesorsen (Waylivra) This triglyceride-reducing drug is a second-generation 2'-O-methoxyethyl (2'-MOE) chimeric antisense therapeutic oligonucleotide (ASO) targeting the messenger RNA for apolipoprotein C3 (apo-CIII). Sequence:
| ASO 20mer Gapmer | 2019.05 | Familial chylomicronaemia syndrome (FCS) (also known as type I hyperlipo- proteinaemia). |
Givosiran (Givlaari)
| siRNA | 2019.11 | Acute Hepatic Porphyrias |
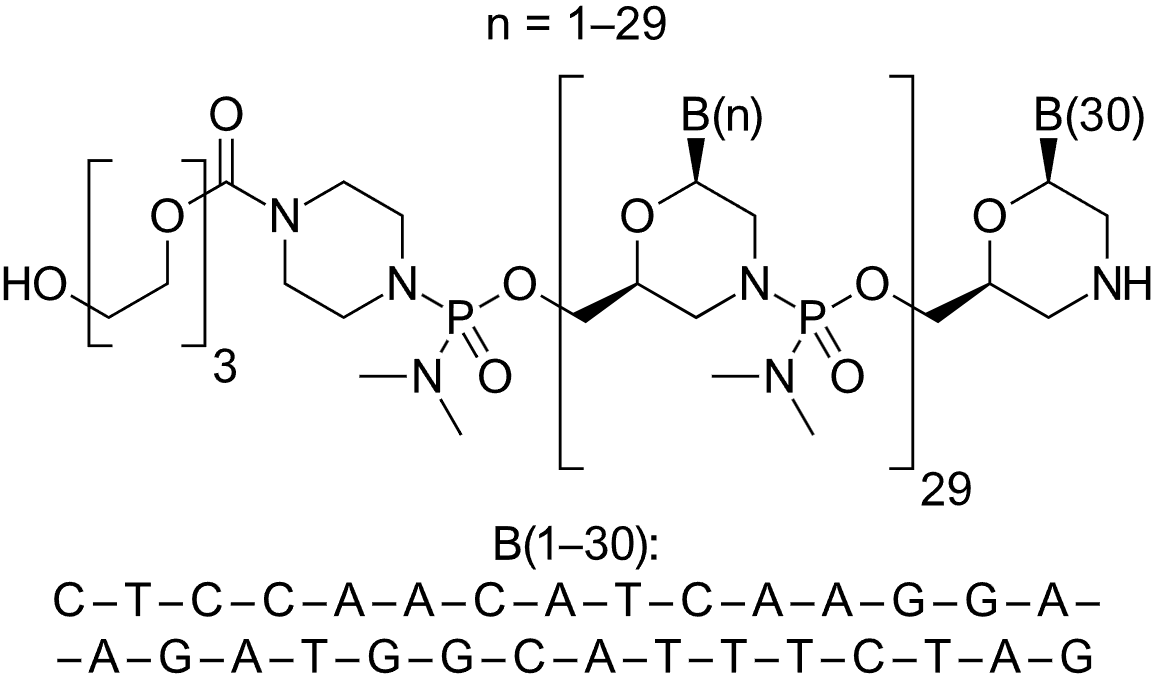
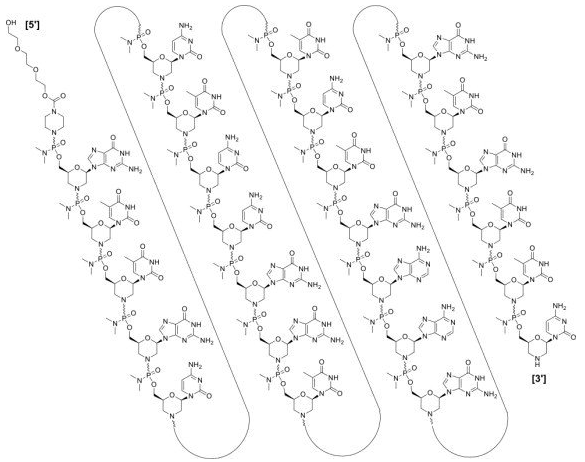
Golodirsen (Vyondys 53) all-P-ambo-[2′,3′-Azanediyl-P-(dimethylamino)-P,2′,3′-trideoxy-2′,3′-seco](2′-N→5′) (G-T-T-G-C-C-T-C-C-G-G-T-T-C-T-G-A-A-G-G-T-G-T-T-C) 5′-{P-[4-({2-[2-(2-hydroxyethoxy)-ethoxy]-ethoxy}-carbonyl)-piperazin-1-yl]-N,N-dimethylphosphonamidate}; Formula: C305H481N138O112P25 Golodirsen, Approval, DB15593, Vyondys-53, Golodirsen, Golodirsen | ASO | 2019.12 | Duchenne Muscular Dystrophy |
Viltolarsen (Viltepso) Morpholino oligonucleotide (PMO) | ASO | 2020.08 | Duchenne Muscular Dystrophy |
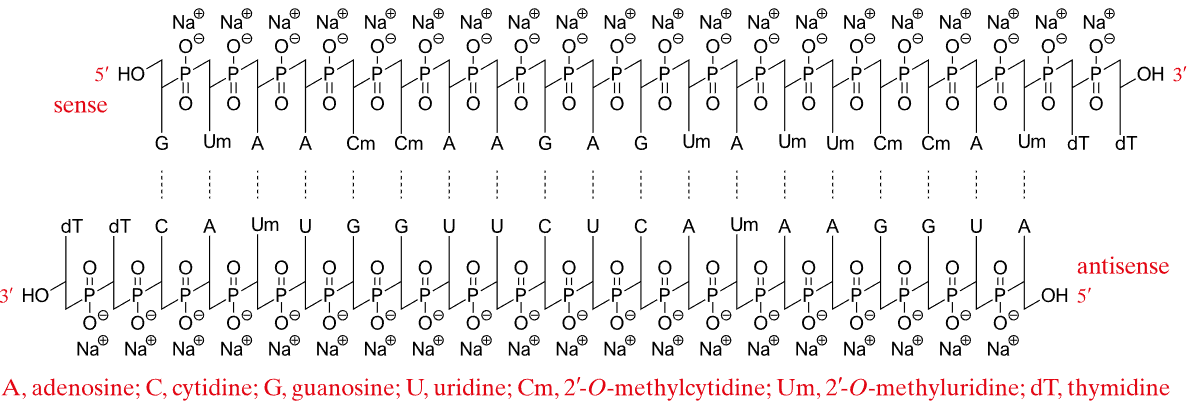
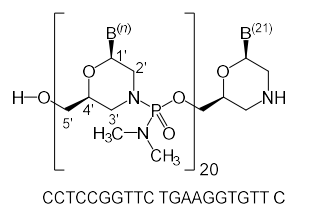
Lumasiran (Oxlumo) Lumasiran sodium: C530H669F10N173O320P43S6Na43, Mw 17,286 Da. | siRNA | 2020.11 | Primary Hyperoxaluria Type 1 (PH1) |
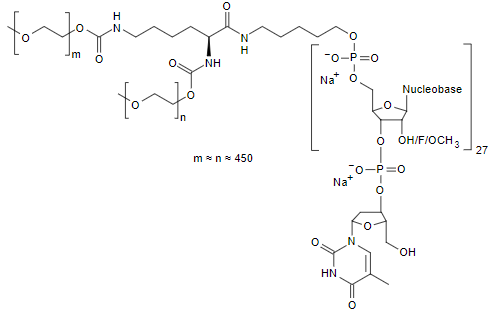
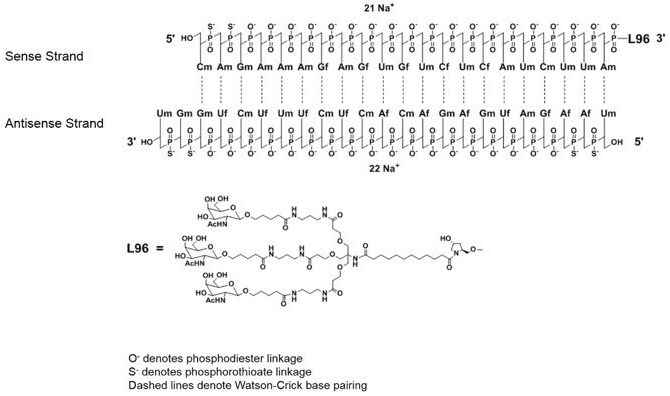
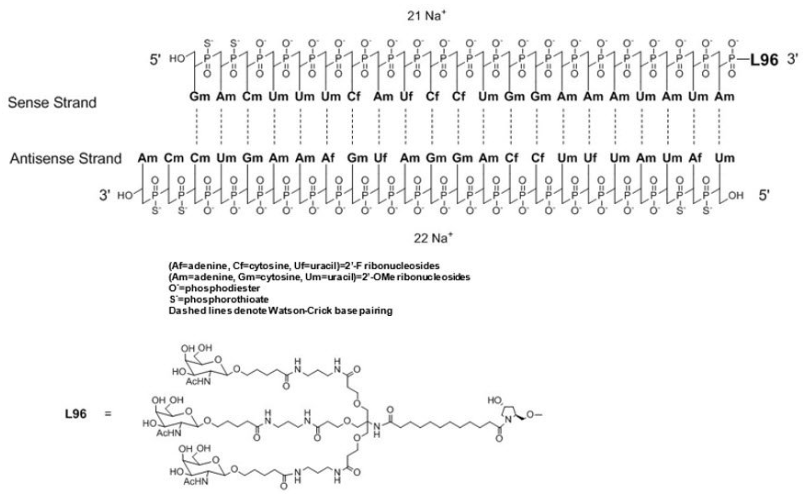
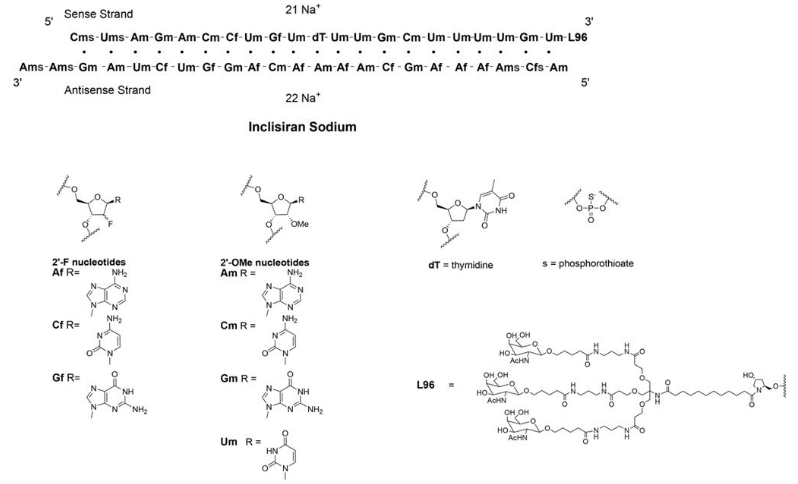
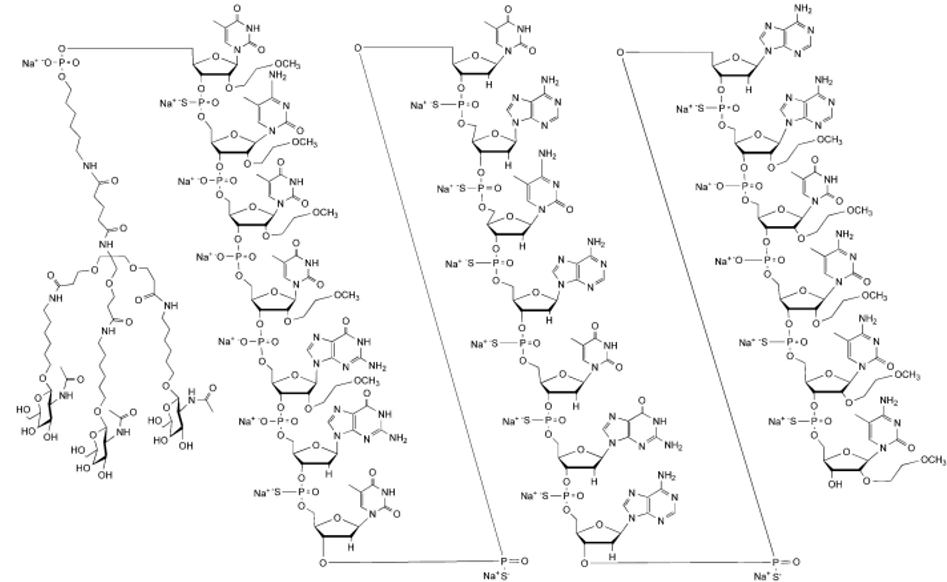
Inclisiran (LeqvioTM) Abbreviations: Af = adenine 2'-F ribonucleotide; Cf = cytosine 2'-F ribonucleotide; Gf = guanine 2'-F ribonucleotide; Am = adenine 2'-OMe ribonucleotide; Cm = cytosine 2'-OMe ribonucleotide; Gm = guanine 2'-OMe ribonucleotide; Um = uracil 2'-OMe ribonucleotide; L96 = triantennary GalNAc (N-acetyl-galactosamine). | siRNA | 2021.12 | Hypercholesterolemia |
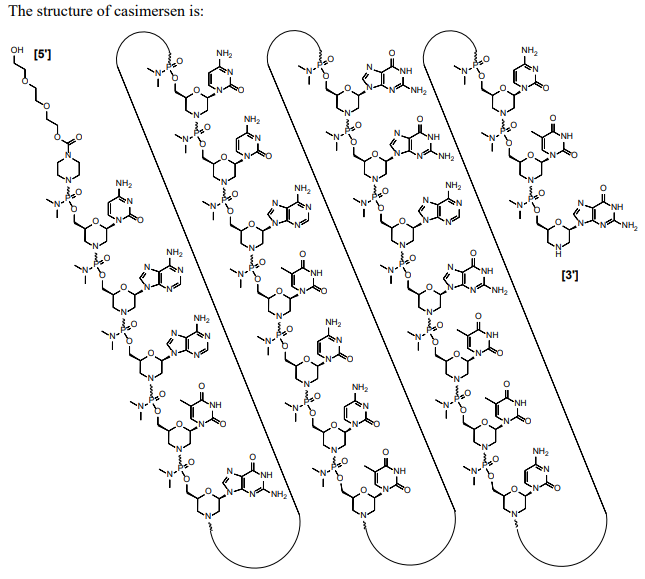
Casimersen (Amondys 45) Sequence: 5'-CAATGCCATCCTGGAGTTCCTG-3'. C268H424N124O95P22. Mw 7584.5 daltons. | ASO | 2021.02 | Duchenne Muscular Dystrophy |
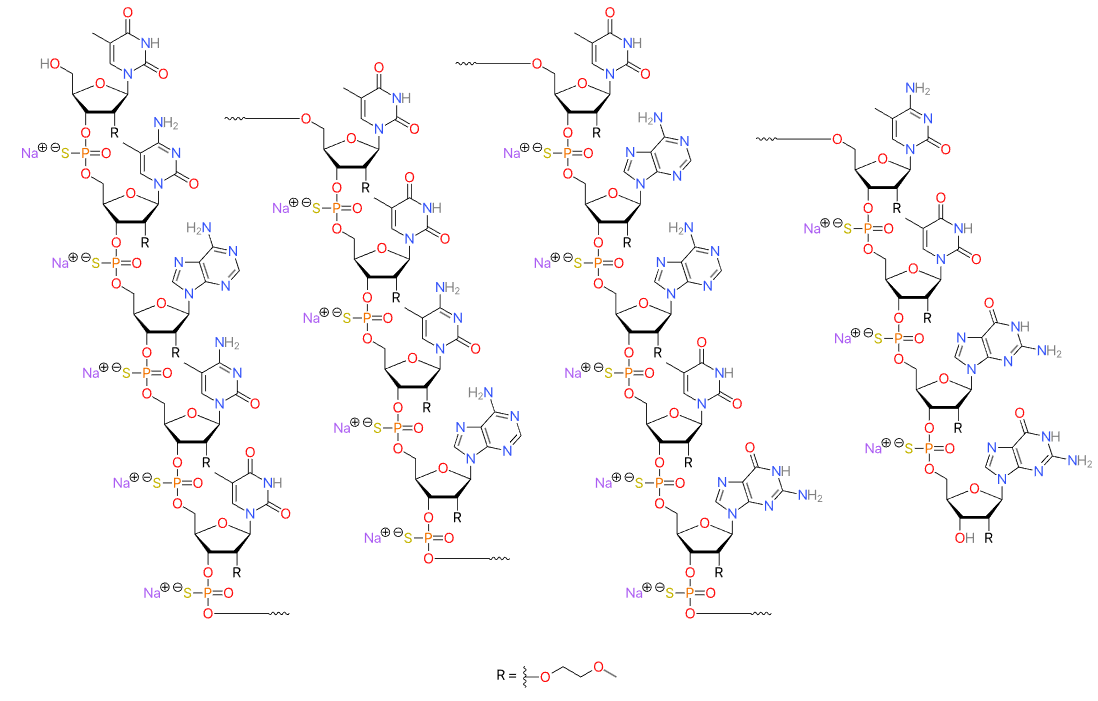
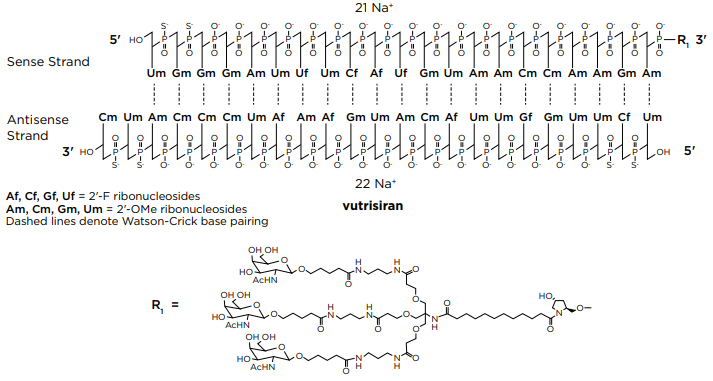
Vutrisiran (Amvuttra) Medication for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults, targeting the mRNA of transthyretin. Vutrisiran sodium: C530H672F9N171Na43O323P43S6; Mw: 17,290 Da. Free acid: C530H715F9N171O323P43S6; Mw: 16,345 Da. Vutrisiran, DB16699, Fda-novel-drug-approvals-june-2022/, AMVUTTRA | siRNA | 2022.06 | TTR, liver |
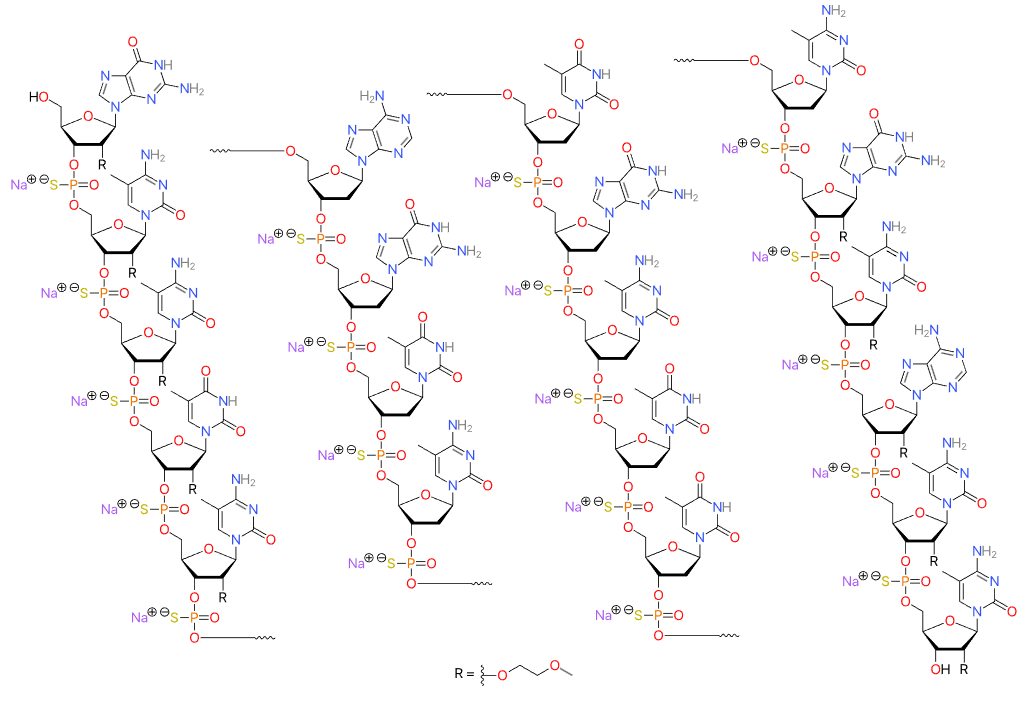
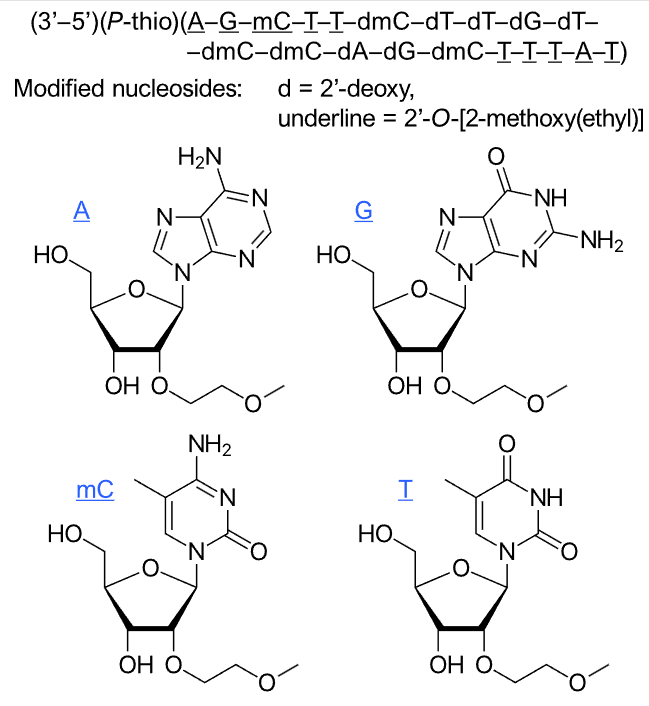
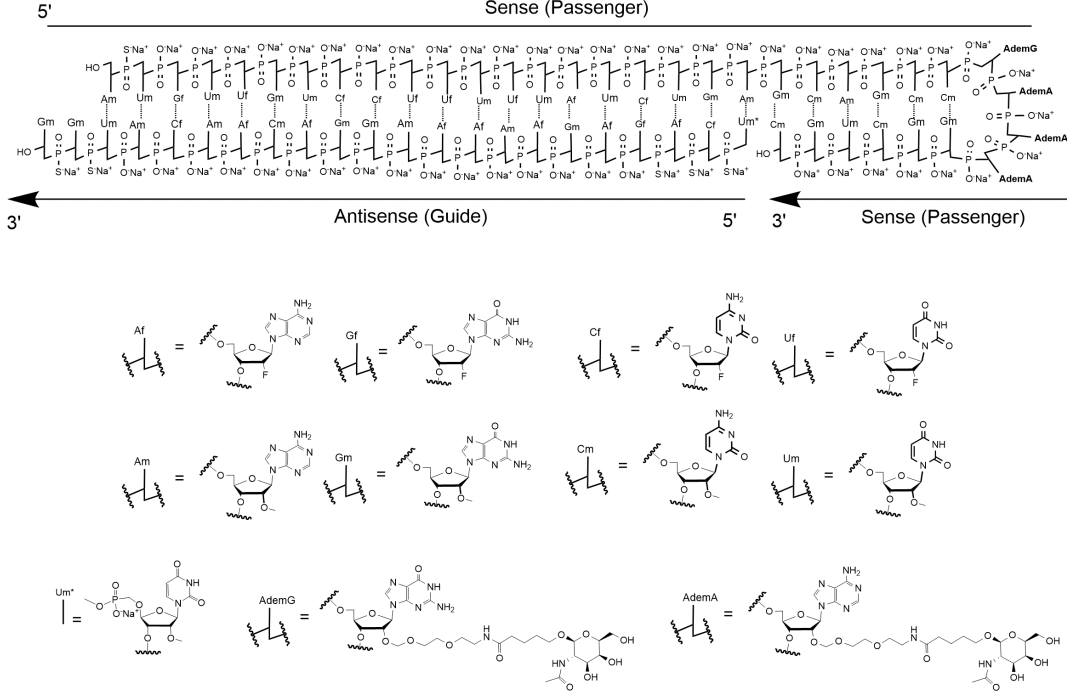
Nedosiran (Rivfloza) Nedosirna sodium: C662H808F19N231O413P57S6Na57, Mw: 22,238 Da, freely soluble in water. DB17635, PMC, Eplontersen, FDA | siRNA | 2023.09 | Primary Hyperoxaluria (PH) |
Eplontersen (Wainua)
| ASO | 2023.12 | Hereditary Transthyretin Amyloidosis |
---...---
" Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including 5'-triphosphate and back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides".
---...---
CpG Oligonucleotides for the Development of Adjuvants and Therapeutics
CpG oligonucleotides (CpG ODNs or CpG) are short single-stranded DNA oligonucleotides containing a cytosine guanine (CpG) motif within their sequence. The “p” in this motif refers to the phosphodiester group linking the two nucleotides.
Oligonucleotides containing unmethylated CpG motifs act as immunostimulants for the innate immune system. Immune-stimulating oligonucleotides (ISOs) activate or stimulate the innate immune system via their interaction with pattern recognition receptors, encode immunostimulatory proteins or peptides, or silence specific genes to block negative regulators of the immune system. Several specific nucleic acids and oligonucleotides can act as immunostimulants.
During viral detection, cellular antiviral defenses sense foreign nucleic acids among abundant self-nucleic acids. This mechanism is known as “immune sensing.” However, an effective antiviral defense requires a balanced process of sensing foreign nucleic acids and ignoring self-nucleic acids.
This balance is accomplished by a multilevel system combining the immune sensing of pathogen-specific nucleic acid structures with specific labeling of self-nucleic acids and nuclease-mediated degradation.
Sensing nucleic acids released from pathogens and damaged or malignant cells is the primary mechanism by which innate immune cells recognize “foreign” substances and activate signaling pathways to initiate their antimicrobial and proinflammatory functions.
TLR9 recognizes unmethylated CpG regions and, when stimulated, activates B cells and human plasmacytoid dendritic cells. The activation results in a potent T helper-1 (Th1)-type immune response and an antitumor response in mouse tumor models and patients. Mammals mainly express TLR9 in subsets of Dendritic Cells and B cells. TLR9 receptors recognize different CpG motifs. Optimal sequences are GTCGTT and GACGTT for human TLR9.
Unmethylated CpG DNA containing CpG-dinucleotides is more common in bacterial genomes than in vertebrate genomes. Methylation at the CG sites generally inhibits the activity of CpG dinucleotides. The CpG motif stimulates immune cells via the toll-like receptor 9 signaling pathway.
Classes of CpG oligonucleotides and their effects (wiki/CpG_ODNs)

Krieg et al. (1995) iteratively determined that the immunostimulatory activity of DNA sequences is restricted to a stretch of 12 to 20 base pairs containing CpG dinucleotides with selective flanking bases with the motif 5′-Pu-Pu-CpG-Pyr-Pyr-3′ as being biologically active.
The diverse functions of nucleic acids and oligonucleotides are critical components in vaccine development and cancer immunotherapy. For example, vaccination of the mucous membranes, the inner lining tissue cells of the nose, mouth, lungs, and stomach can initiate enhanced systemic and mucosal humoral and cellular immune responses, resulting in protection against pathogens, even at a different mucosal site. Also, combining CpG ODNs with radiation and chemotherapy can be effective. For example, treating tumors with CpG ODNs combined with radiation and docetaxel enhances the response and improves the cure rate of murine tumors.
Unfortunately, the instability, poor pharmacokinetics profile, non-specific biodistribution, and difficulty in accessing intracellular targets of CpG oligonucleotides make it challenging to develop CpG oligonucleotide-based therapeutics.
CpG 1018 is an adjuvant used in Heplisav-B vaccine made up of CpG motifs. When CpG 1018 is included in vaccines, it increases the body’s immune response.
Reference
Bauer M., Heeg K., Wagner H. DNA activates human immune cells through a CpG sequence-dependent manner. Immunology. 1999;97:699–705. [PMC]
Chen W, Jiang M, Yu W, Xu Z, Liu X, Jia Q, Guan X, Zhang W. CpG-Based Nanovaccines for Cancer Immunotherapy. Int J Nanomedicine. 2021 Aug 5;16:5281-5299. [PMC]
Jahrsdörfer B, Weiner GJ. CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther. 2008 Mar;3(1):27-32. [PMC]
Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546. [PubMed]
Lin Y-X, Wang Y, Blake S, Yu M, Mei L, Wang H, Shi J, RNA Nanotechnology-Mediated Cancer Immunotherapy, Theranostics, 10 (2020) 281–299. [PMC]
Meng F, Wang J, Yeo Y. Nucleic acid and oligonucleotide delivery for activating innate immunity in cancer immunotherapy. J Control Release. 2022 May;345:586-600. [PMC]
Schlee M, Hartmann G, Discriminating self from non-self in nucleic acid sensing, Nature Reviews Immunology, 16 (2016) 566–580.
Shen T, Zhang Y, Zhou S, Lin S, Zhang X-B, Zhu G, Nucleic Acid Immunotherapeutics for Cancer, ACS Appl Bio Mater, 3 (2020) 2838–2849.
Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10833-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC23500/
---…---
Asthma, CpG DNA and Toll-like Receptor-9
What is the cause of asthma?
Reduced exposure in early life to microbes and microbial products is thought to cause asthma. People who were raised on farms and who attended out-of-home daycare earlier have fewer and less severe atopic disorders. The notion of “atopic disorder” describes a wide range of genetically mediated allergic diseases. The list includes allergic rhinitis, asthma, and atopic dermatitis (AD) associated with heightened T-helper type 2-driven inflammatory responses to common allergens, especially inhaled and food allergens.
The exact causes of asthma are not yet known. Many different reasons may contribute to asthma. Many environmental factors may trigger asthma in people sensitive to this atopic disorder.

(Source: https://simple.wikipedia.org/wiki/Asthma).
Bacterial DNA, which differs from mammalian DNA in the frequency of cytosine-guanine (CpG) dinucleotides, is an immune-stimulating substance. CpG oligonucleotides (CpG ODNs) induce type 1 T helper (Th1) cells to produce Th1 cytokines (interferon-γ, interleukin-2, and tumor necrosis factor-β) to activate macrophages. Macrophages are responsible for cell-mediated immunity and phagocyte-dependent protective responses.
Scientists have speculated that CpG ODNs may help prevent or reverse the white blood cell-linked (eosinophilic) inflammation of atopic asthma. Inducing the regulatory-type responses of T cells and antigen-presenting cells with CpG ODNs is thought to protect against atopic diseases such as asthma. Ongoing clinical trials examine CpG ODNs used alone and as adjuvants for immunotherapy in human populations with atopic diseases.
Bacterial and viral DNA oligodeoxynucleotides containing unmethylated cytosine-guanine (CpG) dinucleotides are found much more frequently than in vertebrates. These strongly immune-active CpG ODNs induce the proliferation and activation of B cells.
In recent decades, research scientists identified additional specific immune responses to CpG dinucleotide DNA in molecular pathways linking recognition of the CpG ligand with its effects. A limited number of human immune cells, most notably plasmacytoid DCs and B cells, constitutively express the receptor for CpG DNA, TLR-9.
CpG DNA enters cells through a process called endocytosis, in which cells absorb external substances by engulfing them with the cell membrane. After entering the cell, TLR-9 recognizes CpG oligonucleotides and translocate them to the nucleus, where they activate the nuclear factor-κB.
The early effects of exposure primarily lead to increased innate immune responses. Activated B cells and plasmacytoid DCs release IL-10, type-I IFNs, IL-12, IFN-inducible protein-10, and other cytokines and chemokines, inducing a regulatory/Th1-oriented inflammatory milieu.
Natural killer cells, T cells, and others that amplify and modulate the immune response react to these molecules. The induction of costimulatory receptors, immunoglobulin isotype switching by B-cells, and the activation of a cascade of cellular responses promoting adaptive immune responses are later effects.
Reference
Akira S. TLR signaling. Curr Top Microbiol Immunol 2006;311:1–16. [PubMed]
Bellanti JA, Settipane RA. The atopic disorders and atopy … "strange diseases" now better defined! Allergy Asthma Proc. 2017 Jul 1;38(4):241-242. [PMC]
Berger A. Th1 and Th2 responses: what are they? BMJ. 2000 Aug 12;321(7258):424. [PMC]
Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, et al. Immunotherapy with a ragweed-Toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med 2006;355:1445–1455. [PubMed]
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000;408:740–745. [PubMed]
Kline JN. Eat dirt: CpG DNA and immunomodulation of asthma. Proc Am Thorac Soc. 2007 Jul;4(3):283-8. [PMC]
Obermeier F, Strauch UG, Dunger N, Grunwald N, Rath HC, Herfarth H, Scholmerich J, Falk W. In vivo CpG DNA/toll-like receptor 9 interaction induces regulatory properties in CD4+CD62L+ T cells which prevent intestinal inflammation in the SCID transfer model of colitis. Gut 2005;54:1428–1436. [PMC] [PubMed]
Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999 Nov;5(4):285-94. [PubMed]
Zeng, G., Zhang, G. & Chen, X. Th1 cytokines, true functional signatures for protective immunity against TB?. Cell Mol Immunol 15, 206–215 (2018). [CMI], [nature]
---...---
Gapmer Antisense Oligonucleotides Interact with Proteins
Gapmers are short deoxyribonucleic acid-based antisense oligonucleotides. Antisense oligonucleotides are chemically modified to enhance the pharmacological properties of RNase H1-antisense oligonucleotides (ASOs). Common modifications of gapmer antisense oligonucleotides include a phosphorothioate (PS) backbone and different 2’-modifications. Usually, each end of an ASO gapmer contains 2’-modifications. The PS backbone is a common component of therapeutic nucleic acids.
Generally, gapmers are designed to hybridize or bind to target ribonucleic acid (RNA) sequences to silence a gene through induced cleavage of the targeted sequence by RNase H.
Intracellular proteins are known to bind antisense oligonucleotides (ASOs) containing phosphorothioate bonds. PS-ASOs are known to bind to plasma proteins, including albumin and α2-macroglobulin, that prevent ASOs from being rapidly discarded through urinary excretion. Compared to PO-ASOs, PS-ASOs enhance cellular uptake both in vitro and in vivo.
Therapeutic PS-ASOs enter cells mainly through endocytic pathways and are released from endocytic particles into the cytosol and nucleus to bind to complementary RNAs via base-pairing. 2′-modifications also affect ASO activity, often by increasing the binding affinity of ASOs to RNA targets.
Liang et al. have identified and characterized more than 50 intracellular proteins interacting with ASOs. However, the mechanism of how ASOs interact with these proteins remains largely unknown.
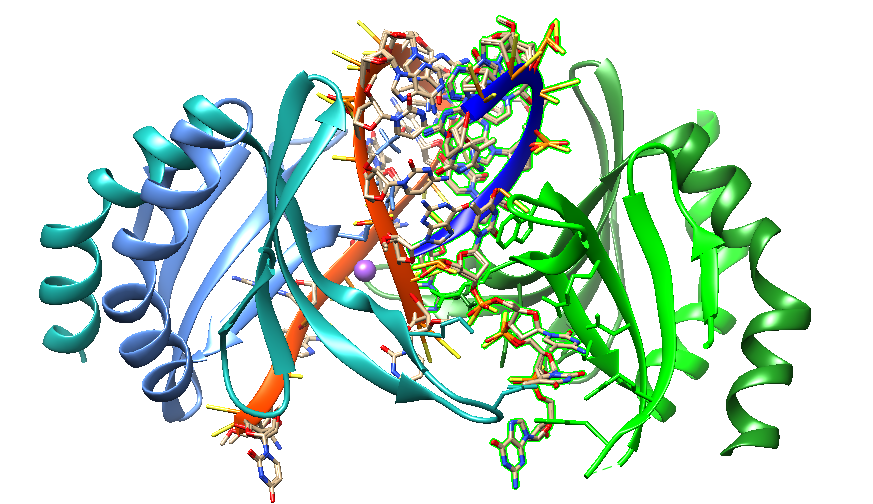
MALAT PS MOE DNA gamer ASO in double stranded form (PDB ID 7ZVN).
2’-MOE PS nucleotides are shown in cyan. PS DNA gapmer in black. All cytidines in the ASO are methylated (5meC). Location of mismatches are highlighted with red dots.
In 2016, Liang et al. studied the protein-binding effects of ASOs containing phosphorothioate (PS) in the backbone and 2′-modifications in the ribose. The researchers utilized affinity selection and competition assays to show that the PS backbone modification dominates protein binding. The study found that compared to a PO-backbone, PS-modified ASOs bind more proteins more tightly. Also, the number of PS-modified nucleotides affects the binding of many proteins to ASOs. ASOs containing less than 10 PS-modified nucleotides interacted with proteins significantly weaker than ASOs with greater PS numbers, and the 2′-modifications in the wings of gapmer ASOs affected protein binding as well.
According to Liang et al., the Hsp90 protein interacts more strongly with PS-ASOs containing locked-nucleic acid (LNA) or constrained-ethyl-bicyclic-nucleic acid ((S)-cEt) modifications than with 2′-O-methoxyethyl (MOE). Also, ASOs bind to the mid-domain of the Hsp90 protein. ASOs containing the hydrophobic 2′-modifications (S)-cEt or LNA in the 5′-wing interact with Hsp90.
Reduction of the Hsp90 protein decreased the activity of PS-ASOs with 5′-LNA or 5′-cEt wings but not with 5′-MOE wings. This study by Liang et al. indicates that the Hsp90 protein enhances the activity of ASOs modified with PS and LNA moieties or PS and (S)-cEt moieties, suggesting that different chemical modifications can improve the therapeutic efficacy of PS-ASOs.
In 2020, Hyjek-Składanowska reported a crystal structure-based model of the DNA-binding domain of the PC4 protein in complex with a PS 2′-OMe DNA gapmer. In the structure, each PC4 dimer contains two DNA-binding interfaces. The 5′-terminal 2′-OMe PS flank is bound by one interface. The regular PS DNA central part is bound in opposite polarity as a hairpin-like structure by the other interface. Binding to the ASO induces the formation of a dimer of dimers of PC4. The base pairing between regions of the ASO stabilizes the binding to each PC4 dimer. The PS nucleic acid interacts with the protein through electrostatic and hydrophobic contacts. NanoBRET binding assay confirmed these contacts.
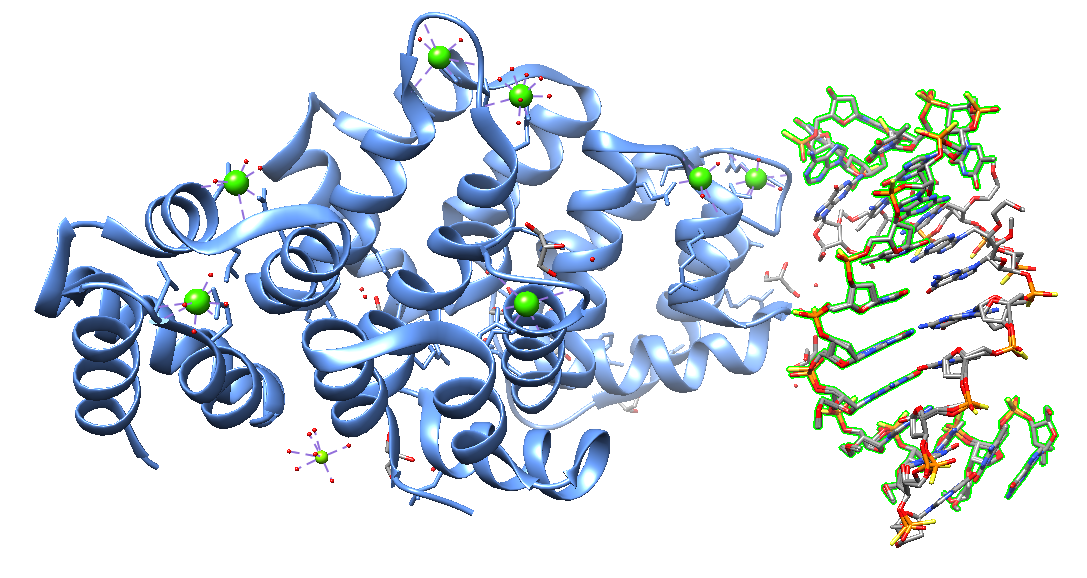
Human Transcription Cofactor PC4 DNA-binding domain in complex with full phosphorothioate 5-10-5 2'-O-methyl DNA gapmer antisense oligonucleotide [6YCS].
Hyjek-Składanowska et al. suggest that this structure provides insights into the molecular forces governing the interactions of PS ASOs with cellular proteins. Furthermore, it provides a potential model for how these interactions can cause cellular toxicity. Pandey et al. (2021) state that the interaction between human positive coactivator 4 (PC4) and the tumor suppressor protein p53 is crucial in initiating apoptosis. PC4 is an abundant nuclear protein. Since PC4 assisted-p53-dependent apoptosis may play a central role in certain neurodegenerative diseases, disrupting p53-PC4 interactions may be a promising drug target for specific disease pathologies.
More recently, in 2023, Hyjek-Składanowska et al. reported the crystal structure of human Annexin A2 in complex with a phosphorothioate 5-10 2”-MOE DNA gapmer ASO. The research group co-crystallized the C-terminal domain (CTD) core of annexin A2 (AnxA2) with a phosphorothioate ( PS) 2′-methoxyethyl (MOE) DNA gapmer ASO. The crystal structure revealed that unique hydrophobic interactions between PS groups, lysine and arginine residues account for the enhanced affinity of PS ASOs to the protein surface. These results demonstrate that this interaction mechanism appears to be a general phenomenon observed not only for nucleic acid-binding proteins but may also explain interactions of ASOs proteins.
Crystal structure of human Annexin A2 in complex with full phosphorothioate 5-10 2'-methoxyethyl DNA gapmer antisense oligonucleotide solved at 1.87 Å resolution [7ZVN].
The annexin A (ANXA) protein family is a well-known tissue-specific multigene family encoding calcium (Ca2+) phospholipid-binding proteins. ANXA proteins play essential roles in cancer progression, proliferation, invasion, and metastasis in several diseases.
Reference
Crooke ST, Vickers TA, Liang XH. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020 Jun 4;48(10):5235-5253. [PMC]
Hyjek-Składanowska M, Vickers TA, Napiórkowska A, Anderson BA, Tanowitz M, Crooke ST, Liang XH, Seth PP, Nowotny M. Origins of the Increased Affinity of Phosphorothioate-Modified Therapeutic Nucleic Acids for Proteins. J Am Chem Soc. 2020 Apr 22;142(16):7456-7468. [PubMed][pdb/6ycs]
Hyjek-Składanowska M, Anderson BA, Mykhaylyk V, Orr C, Wagner A, Poznański JT, Skowronek K, Seth P, Nowotny M. Structures of annexin A2-PS DNA complexes show dominance of hydrophobic interactions in phosphorothioate binding. Nucleic Acids Res. 2023 Feb 22;51(3):1409-1423. [PMC]
Liang XH, Shen W, Sun H, Kinberger GA, Prakash TP, Nichols JG, Crooke ST. Hsp90 protein interacts with phosphorothioate oligonucleotides containing hydrophobic 2'-modifications and enhances antisense activity. Nucleic Acids Res. 2016 May 5;44(8):3892-907. [PMC]
Pandey B, Dev A, Chakravorty D, Bhandare VV, Polley S, Roy S, Basu G. Insights on the disruption of the complex between human positive coactivator 4 and p53 by small molecules. Biochem Biophys Res Commun. 2021 Nov 12;578:15-20. [PubMed]
Zhang H, Zhang Z, Guo T, Chen G, Liu G, Song Q, Li G, Xu F, Dong X, Yang F, Cao C, Zhong D, Li S, Li Y, Wang M, Li B, Yang L. Annexin A protein family: Focusing on the occurrence, progression and treatment of cancer. Front Cell Dev Biol. 2023 Mar 3;11:1141331. [PMC]
---...---
" Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including 5'-triphosphate and back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides".
---...---
Enhancer RNAs add a crucial regulatory layer to the genome
Enhancer RNAs add a crucial regulatory layer to the genome. Kim et al. (2010) identified a new class of RNAs called enhancer RNAs or eRNAs. Enhancers are cis-regulatory gene elements and are crucial for controlling temporal and cell-type specific patterns of gene expression.
Several classes of non-coding RNAs (ncRNAs), including microRNAs (miRNAs) and long non-coding RNAs (ncRNAs), play diverse roles in post-transcriptional regulation of mRNA stability and epigenetic control of chromatin activities.
Enhancer RNA or eRNA are RNAs transcribed by RNA polymerase II (RNAPII) from the domain of transcription enhancers. They are a class of short, non-coding RNAs transcribed from DNA enhancer regions, 50 to 2,000 nucleotides in length. Enhancer regions are bidirectionally transcribed into enhancer RNAs (eRNAs). As a response to signaling pathways, intergenic DNA elements known as enhancers regulate target gene transcription. Enhancers interact with promotors over large genomic distances. Enhancers contain binding sites for transcription factors promoting RNA polymerase II (RNAPII) recruitment and activation. Enhancers carry unique epigenetic marks, distinguishing them from promoters.
These regulatory elements also have an open chromatin conformation that increases accessibility to transcription factors and RNAPII. Rahman et al. recently reported that eRNAs are localized exclusively in the nucleus. The induction of eRNAs occurs with similar kinetics as that of target mRNAs. eRNAs are beginning to develop mostly as enhancers. The steady-state levels of eRNAs remain lower than those of their related mRNAs and RNAs at the single-allele level. Also, eRNAs are rarely co-expressed with their target loci. However, active gene transcription does not require continuous transcription of eRNAs or the accumulation of eRNAs at enhancers.
Recently developed genome-wide sequencing methods allow studying stimulus-dependent enhancer functions in tissue cells. Kim et al. (2010) found that the level of eRNA expression at neuronal enhancers positively correlates with the level of mRNA synthesis at nearby genes. These observations suggest that eRNA synthesis occurs specifically at enhancers that actively promote mRNA synthesis and that a widespread mechanism of enhancer activation involves RNAPII binding and eRNA synthesis.
Kim et al. suggested that establishing and maintaining the chromatin landscape at enhancers requires eRNA synthesis for enhancer function. It is also possible that the eRNA transcripts are functionally crucial by themselves. A typical gene transcribed by RNA polymerase II has a promotor that extends upstream from the site where transcription is initiated (Figure 1).
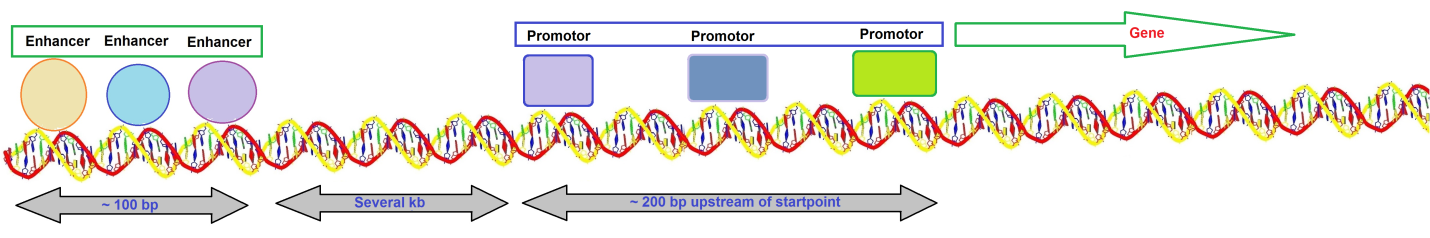
Figure 1: Overview of a typical gene transcribed by RNA polymerase II.
The promotor contains several short sequence elements that bind transcription factors, <10 base pairs (bp) in length, and promotors are dispersed over a sequence region >200 bp. Enhancers contain a more closely packed array of elements that bind transcription factors. Enhancer regions appear to be located at a distance of several kilobases (kb). The DNA duplex may be coiled or rearranged such that transcription factors at the promotor and the enhancer interact to form a large protein-DNA complex.
Enhancers contain bidirectional elements that allow assisting initiation. The presence of a promotor increases its activity. The enhancer is located distinct from the promotor, and its position relative to the promotor can vary substantially. An enhancer can stimulate any promotor placed in its vicinity. Enhancers often show redundancy in function. DNA must be able to form a loop structure if proteins bound at an enhancer several kb distant from a promotor interact directly with proteins bound in the vicinity of the starting point such that the enhancer and promotor are near to each other. Enhancers may function by bringing proteins into the area of the promotor.
Methods that allow analysis of eRNAs:
- Reverse transcription-PCR (RT-PCR)
- RNA fluorescence in situ hybridization (RNA-FISH)
- RNA polymerase II chromatin immunoprecipitation coupled with high-throughput sequencing (RNAPII ChIP–seq)
- Global run-on sequencing (GRO-seq)
- 5′GRO-seq or GRO-cap
- BruUV-seq
- RNA-seq (total)
- RNA-seq (poly(A))
- Cap analysis of gene expression (CAGE) followed by deep sequencing
- Chromatin- bound RNA-seq
- RNA-Seq in isolated 'transcription factories'
- Native elongating transcript sequencing (NET-seq)
- RNA capture sequencing (CaptureSeq)
- Chromatin isolation by RNA purification (ChIRP-seq)
Reference
Bose DA, Berger SL. eRNA binding produces tailored CBP activity profiles to regulate gene expression. RNA Biol. 2017 Dec 2;14(12):1655-1659. [PMC]
Jourdain AA, Koppen M, Rodley CD, Maundrell K, Gueguen N, Reynier P, Guaras AM, Enriquez JA, Anderson P, Simarro M, Martinou JC. A mitochondria-specific isoform of FASTK is present in mitochondrial RNA granules and regulates gene expression and function. Cell Rep. 2015 Feb 24;10(7):1110-21. [Cell Reports]
Kim, T.-K., Hemberg, M., Gray, J. M., Costa, A. M., Bear, D. M., Wu, J., … Greenberg, M. E. (2010). Widespread transcription at neuronal activity-regulated enhancers. Nature, 465(7295), 182–187. [Nature]
Lewin, Benjamin; Genes VII chapter 20, 2000.
Li, W., Notani, D. & Rosenfeld, M. Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat Rev Genet 17, 207–223 (2016). [Nature Reviews genetics]
Rahman, S., Zorca, C. E., Traboulsi, T., Noutahi, E., Krause, M. R., Mader, S., & Zenklusen, D. (2017). Single-cell profiling reveals that eRNA accumulation at enhancer–promoter loops is not required to sustain transcription. Nucleic Acids Research, 45(6), 3017–3030. [NAR]
---...---
Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including 5'-triphosphate and back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides".
---...---